IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass
- PMID: 21670317
- PMCID: PMC3896980
- DOI: 10.4049/jimmunol.1003986
IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass
Abstract
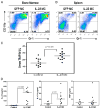
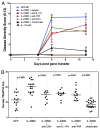
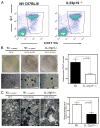
The role of IL-23 in the development of arthritis and bone metabolism was studied using systemic IL-23 exposure in adult mice via hydrodynamic delivery of IL-23 minicircle DNA in vivo and in mice genetically deficient in IL-23. Systemic IL-23 exposure induced chronic arthritis, severe bone loss, and myelopoiesis in the bone marrow and spleen, which resulted in increased osteoclast differentiation and systemic bone loss. The effect of IL-23 was partly dependent on CD4(+) T cells, IL-17A, and TNF, but could not be reproduced by overexpression of IL-17A in vivo. A key role in the IL-23-induced arthritis was made by the expansion and activity of myeloid cells. Bone marrow macrophages derived from IL-23p19(-/-) mice showed a slower maturation into osteoclasts with reduced tartrate-resistant acid phosphatase-positive cells and dentine resorption capacity in in vitro osteoclastogenesis assays. This correlated with fewer multinucleated osteoclast-like cells and more trabecular bone volume and number in 26-wk-old male IL-23p19(-/-) mice compared with control animals. Collectively, our data suggest that systemic IL-23 exposure induces the expansion of a myeloid lineage osteoclast precursor, and targeting IL-23 pathway may combat inflammation-driven bone destruction as observed in rheumatoid arthritis and other autoimmune arthritides.
Figures


References
-
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. - PubMed
-
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. - PubMed
-
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. - PubMed
-
- Li J, Sarosi I, Yan X-Q, Morony S, Capparelli C, Tan H-L, McCabe S, Elliott R, Scully S, Van G, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000;97:1566–1571. - PMC - PubMed
-
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

