Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts
- PMID: 21670498
- PMCID: PMC3223819
- DOI: 10.1172/JCI43578
Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts
Abstract
Hutchinson-Gilford progeria syndrome (HGPS), a devastating premature aging disease, is caused by a point mutation in the lamin A gene (LMNA). This mutation constitutively activates a cryptic splice donor site, resulting in a mutant lamin A protein known as progerin. Recent studies have demonstrated that progerin is also produced at low levels in normal human cells and tissues. However, the cause-and-effect relationship between normal aging and progerin production in normal individuals has not yet been determined. In this study, we have shown in normal human fibroblasts that progressive telomere damage during cellular senescence plays a causative role in activating progerin production. Progressive telomere damage was also found to lead to extensive changes in alternative splicing in multiple other genes. Interestingly, elevated progerin production was not seen during cellular senescence that does not entail telomere shortening. Taken together, our results suggest a synergistic relationship between telomere dysfunction and progerin production during the induction of cell senescence, providing mechanistic insight into how progerin may participate in the normal aging process.
Figures
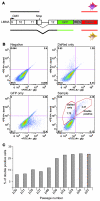
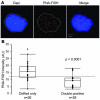
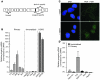

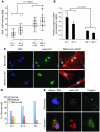

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

