Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions
- PMID: 21670501
- PMCID: PMC3223823
- DOI: 10.1172/JCI44489
Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions
Abstract
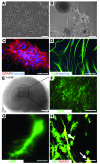
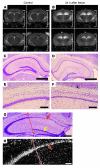
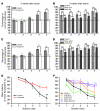
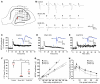
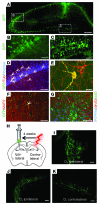
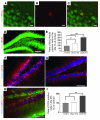
Stem cell-based therapy has been proposed as a potential means of treatment for a variety of brain disorders. Because ethical and technical issues have so far limited the clinical translation of research using embryonic/fetal cells and neural tissue, respectively, the search for alternative sources of therapeutic stem cells remains ongoing. Here, we report that upon transplantation into mice with chemically induced hippocampal lesions, human olfactory ecto-mesenchymal stem cells (OE-MSCs) - adult stem cells from human nasal olfactory lamina propria - migrated toward the sites of neural damage, where they differentiated into neurons. Additionally, transplanted OE-MSCs stimulated endogenous neurogenesis, restored synaptic transmission, and enhanced long-term potentiation. Mice that received transplanted OE-MSCs exhibited restoration of learning and memory on behavioral tests compared with lesioned, nontransplanted control mice. Similar results were obtained when OE-MSCs were injected into the cerebrospinal fluid. These data show that OE-MSCs can induce neurogenesis and contribute to restoration of hippocampal neuronal networks via trophic actions. They provide evidence that human olfactory tissue is a conceivable source of nervous system replacement cells. This stem cell subtype may be useful for a broad range of stem cell-related studies.
Figures

Similar articles
-
Isolation and characterization of olfactory ecto-mesenchymal stem cells from eight mammalian genera.BMC Vet Res. 2018 Jan 17;14(1):17. doi: 10.1186/s12917-018-1342-2. BMC Vet Res. 2018. PMID: 29343270 Free PMC article.
-
Isolating nasal olfactory stem cells from rodents or humans.J Vis Exp. 2011 Aug 22;(54):2762. doi: 10.3791/2762. J Vis Exp. 2011. PMID: 21876529 Free PMC article.
-
Olfactory mucosa stem cells: An available candidate for the treatment of the Parkinson's disease.J Cell Physiol. 2019 Dec;234(12):23763-23773. doi: 10.1002/jcp.28944. Epub 2019 Jun 7. J Cell Physiol. 2019. PMID: 31173364
-
Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories?Neurosurg Clin N Am. 2007 Jan;18(1):105-13, x. doi: 10.1016/j.nec.2006.10.008. Neurosurg Clin N Am. 2007. PMID: 17244558 Free PMC article. Review.
-
Olfactory Ecto-Mesenchymal Stem Cells in Modeling and Treating Alzheimer's Disease.Int J Mol Sci. 2024 Aug 3;25(15):8492. doi: 10.3390/ijms25158492. Int J Mol Sci. 2024. PMID: 39126059 Free PMC article. Review.
Cited by
-
Olfactory ecto-mesenchymal stem cells possess immunoregulatory function and suppress autoimmune arthritis.Cell Mol Immunol. 2016 May;13(3):401-8. doi: 10.1038/cmi.2015.82. Epub 2015 Sep 21. Cell Mol Immunol. 2016. PMID: 26388237 Free PMC article.
-
Transplantation of olfactory mucosa mesenchymal stromal cells repairs spinal cord injury by inducing microglial polarization.Spinal Cord. 2024 Aug;62(8):429-439. doi: 10.1038/s41393-024-01004-6. Epub 2024 Jun 7. Spinal Cord. 2024. PMID: 38849489
-
Olfactory Ecto-mesenchymal Stem Cell-derived Exosomes Ameliorate Murine Sjögren's Syndrome via Suppressing Tfh Cell Response.Rheumatol Immunol Res. 2022 Dec 31;3(4):198-207. doi: 10.2478/rir-2022-0035. eCollection 2022 Dec. Rheumatol Immunol Res. 2022. PMID: 36879843 Free PMC article.
-
Priming TLR3 and TLR4 in human adipose- and olfactory mucosa-derived mesenchymal stromal cells and comparison of their cytokine secretions.Cytotechnology. 2020 Feb;72(1):57-68. doi: 10.1007/s10616-019-00357-8. Epub 2020 Jan 2. Cytotechnology. 2020. PMID: 31898754 Free PMC article.
-
Human olfactory mesenchymal stromal cells co-expressing horizontal basal and ensheathing cell proteins in culture.Biomedica. 2020 Mar 1;40(1):72-88. doi: 10.7705/biomedica.4762. Biomedica. 2020. PMID: 32220165 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

