Scleroderma-like properties of skin from caveolin-1-deficient mice: implications for new treatment strategies in patients with fibrosis and systemic sclerosis
- PMID: 21670602
- PMCID: PMC3154363
- DOI: 10.4161/cc.10.13.16227
Scleroderma-like properties of skin from caveolin-1-deficient mice: implications for new treatment strategies in patients with fibrosis and systemic sclerosis
Abstract
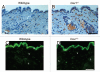
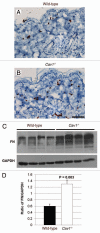
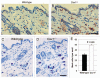
Caveolin-1 (Cav-1), the principal structural component of caveolae, participates in the pathogenesis of several fibrotic diseases, including systemic sclerosis (SSc). Interestingly, affected skin and lung samples from patients with SSc show reduced levels of Cav-1, as compared to normal skin. In addition, restoration of Cav-1 function in skin fibroblasts from SSc patients reversed their pro-fibrotic phenotype. Here, we further investigated whether Cav-1 mice are a useful pre-clinical model for studying the pathogenesis of SSc. For this purpose, we performed quantitative transmission electron microscopy, as well as biochemical and immuno-histochemical analysis, of the skin from Cav-1 (-/-) null mice. Using these complementary approaches, we now show that skin from Cav-1 null mice exhibits many of the same characteristics as SSc skin from patients, including a decrease in collagen fiber diameter, increased tensile strength, and stiffness, as well as mononuclear cell infiltration. Furthermore, an increase in autophagy/mitophagy was observed in the stromal cells of the dermis from Cav-1 (-/-) mice. These findings suggest that changes in cellular energy metabolism (e.g., a shift towards aerobic glycolysis) in these stromal cells may be a survival mechanism in this "hostile" or pro-inflammatory microenvironment. Taken together, our results demonstrate that Cav-1 (-/-) null mice are a valuable new pre-clinical model for studying scleroderma. Most importantly, our results suggest that inhibition of autophagy and/or aerobic glycolysis may represent a new promising therapeutic strategy for halting fibrosis in SSc patients. Finally, Cav-1 (-/-) null mice are also a pre-clinical model for a "lethal" tumor micro-environment, possibly explaining the link between fibrosis, tumor progression, and cancer metastasis.
Figures




Comment in
-
Caveolin-1: a new therapeutic target in tissue fibrosis and scleroderma?Cell Cycle. 2011 Nov 1;10(21):3629. doi: 10.4161/cc.10.21.18033. Epub 2011 Nov 1. Cell Cycle. 2011. PMID: 22052358 No abstract available.
References
-
- Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. - PubMed
-
- Jimenez SA, Bashey RI. Collagen synthesis by scleroderma fibroblasts in culture. Arthritis Rheum. 1977;20:902–903. - PubMed
-
- Takeda K, Hatamochi A, Ueki H, Nakata M, Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Invest Dermatol. 1994;103:359–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR050950/AR/NIAMS NIH HHS/United States
- R01 CA098779/CA/NCI NIH HHS/United States
- R01-CA-120876/CA/NCI NIH HHS/United States
- R01 CA120876/CA/NCI NIH HHS/United States
- R01-AR-055660/AR/NIAMS NIH HHS/United States
- R01-CA-080250/CA/NCI NIH HHS/United States
- R01 CA047282/CA/NCI NIH HHS/United States
- R01 CA39481/CA/NCI NIH HHS/United States
- R01 CA080250/CA/NCI NIH HHS/United States
- P30 AR050950/AR/NIAMS NIH HHS/United States
- R01 CA039481/CA/NCI NIH HHS/United States
- R01 CA120975/CA/NCI NIH HHS/United States
- R01-CA-098779/CA/NCI NIH HHS/United States
- R01 AR055660/AR/NIAMS NIH HHS/United States
- R01 CA47282/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
