Mechanisms regulating cilia growth and cilia function in endothelial cells
- PMID: 21671118
- PMCID: PMC11115144
- DOI: 10.1007/s00018-011-0744-0
Mechanisms regulating cilia growth and cilia function in endothelial cells
Abstract
The primary cilium is an important sensory organelle present in most mammalian cells. Our current studies aim at examining intracellular molecules that regulate cilia length and/or cilia function in vitro and ex vivo. For the first time, we show that intracellular cAMP and cAMP-dependent protein kinase (PKA) regulate both cilia length and function in vascular endothelial cells. Although calcium-dependent protein kinase modulates cilia length, it does not play a significant role in cilia function. Cilia length regulation also involves mitogen-activated protein kinase (MAPK), protein phosphatase-1 (PP-1), and cofilin. Furthermore, cofilin regulates cilia length through actin rearrangement. Overall, our study suggests that the molecular interactions between cilia function and length can be independent of one another. Although PKA regulates both cilia length and function, changes in cilia length by MAPK, PP-1, or cofilin do not have a direct correlation to changes in cilia function. We propose that cilia length and function are regulated by distinct, yet complex intertwined signaling pathways.
Figures
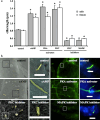
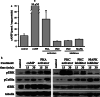

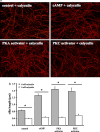
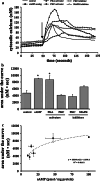

References
-
- Nauli SM, Haymour HS, AbouAlaiwi WA, Lo ST, Nauli AM (2011) Primary cilia are mechanosensory organelles in vestibular tissues. In: Mechanosensitivity and mechanotransduction, Chap 14. Springer, Berlin Heidelberg New York. ISBN: 978-90-481-9880-1
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

