Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency
- PMID: 21671801
- PMCID: PMC3205803
- DOI: 10.1089/hum.2011.069
Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency
Abstract
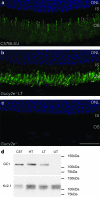
Leber congenital amaurosis (LCA) is a severe retinal dystrophy manifesting from early infancy as poor vision or blindness. Loss-of-function mutations in GUCY2D cause LCA1 and are one of the most common causes of LCA, accounting for 20% of all cases. Human GUCY2D and mouse Gucy2e genes encode guanylate cyclase-1 (GC1), which is responsible for restoring the dark state in photoreceptors after light exposure. The Gucy2e(-/-) mouse shows partially diminished rod function, but an absence of cone function before degeneration. Although the cones appear morphologically normal, they exhibit mislocalization of proteins involved in phototransduction. In this study we tested the efficacy of an rAAV2/8 vector containing the human rhodopsin kinase promoter and the human GUCY2D gene. Following subretinal delivery of the vector in Gucy2e(-/-) mice, GC1 protein was detected in the rod and cone outer segments, and in transduced areas of retina cone transducin was appropriately localized to cone outer segments. Moreover, we observed a dose-dependent restoration of rod and cone function and an improvement in visual behavior of the treated mice. Most importantly, cone preservation was observed in transduced areas up to 6 months post injection. To date, this is the most effective rescue of the Gucy2e(-/-) mouse model of LCA and we propose that a vector, similar to the one used in this study, could be suitable for use in a clinical trial of gene therapy for LCA1.
Figures






Comment in
-
Fighting blindness with adeno-associated virus serotype 8.Hum Gene Ther. 2011 Oct;22(10):1169-70. doi: 10.1089/hum.2011.2521. Hum Gene Ther. 2011. PMID: 22044092 No abstract available.
References
-
- Acland G.M. Aguirre G.D. Ray J., et al. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001;28:92–95. - PubMed
-
- Ali R.R. Sarra G.M. Stephens C., et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat. Genet. 2000;25:306–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

