From in vivo gene targeting of oestrogen receptors to optimization of their modulation in menopause
- PMID: 21671899
- PMCID: PMC3252966
- DOI: 10.1111/j.1476-5381.2011.01538.x
From in vivo gene targeting of oestrogen receptors to optimization of their modulation in menopause
Abstract
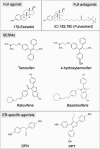
The ancestral status of oestrogen receptor (ER) in the family of the steroid receptors has probably contributed to the pleiotropic actions of oestrogens, and in particular, that of 17β-oestradiol (E2). Indeed, in addition to their well-described role in sexual development and reproduction, they influence most of the physiological processes. The pathophysiological counterpart of these actions includes prevention of osteoporosis, atheroma and type 2 diabetes, and also the promotion of uterus and breast cancer growth. Thus, the major challenge consists in uncoupling some beneficial actions from other deleterious ones, that is, selective ER modulation. Tamoxifen and raloxifene are already used, as they prevent the recurrence of breast cancer and mimic oestrogen action mainly on bone. Both E2 and tamoxifen exhibit a proliferative and, thus, a protumoural action on the endometrium. Activation of ERα and ERβ regulates target gene transcription (genomic action) through two independent activation functions, AF-1 and AF-2, but can also elicit rapid membrane-initiated steroid signals. In the present review, we attempted to summarize recent advances provided by the in vivo molecular 'dissection' of ERα, allowing the uncoupling of some of its actions and potentially paving the way to optimized selective ER modulators.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

References
-
- Akhter MP, Lappe JM, Davies KM, Recker RR. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41:111–116. - PubMed
-
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. - PubMed
-
- Anderson GL, Chlebowski RT, Rossouw JE, Rodabough RJ, McTiernan A, Margolis KL, et al. Prior hormone therapy and breast cancer risk in the Women's Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55:103–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

