Dosing equation for tacrolimus using genetic variants and clinical factors
- PMID: 21671989
- PMCID: PMC3244642
- DOI: 10.1111/j.1365-2125.2011.04039.x
Dosing equation for tacrolimus using genetic variants and clinical factors
Abstract
Aim: To develop a dosing equation for tacrolimus, using genetic and clinical factors from a large cohort of kidney transplant recipients. Clinical factors and six genetic variants were screened for importance towards tacrolimus clearance (CL/F).
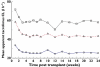
Methods: Clinical data, tacrolimus troughs and corresponding doses were collected from 681 kidney transplant recipients in a multicentre observational study in the USA and Canada for the first 6 months post transplant. The patients were genotyped for 2,724 single nucleotide polymorphisms using a customized Affymetrix SNP chip. Clinical factors and the most important SNPs (rs776746, rs12114000, rs3734354, rs4926, rs3135506 and rs2608555) were analysed for their influence on tacrolimus CL/F.
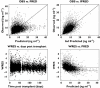
Results: The CYP3A5*1 genotype, days post transplant, age, transplant at a steroid sparing centre and calcium channel blocker (CCB) use significantly influenced tacrolimus CL/F. The final model describing CL/F (l h(-1)) was: 38.4 ×[(0.86, if days 6-10) or (0.71, if days 11-180)]×[(1.69, if CYP3A5*1/*3 genotype) or (2.00, if CYP3A5*1/*1 genotype)]× (0.70, if receiving a transplant at a steroid sparing centre) × ([age in years/50](-0.4)) × (0.94, if CCB is present). The dose to achieve the desired trough is then prospectively determined using the individuals CL/F estimate.
Conclusions: The CYP3A5*1 genotype and four clinical factors were important for tacrolimus CL/F. An individualized dose is easily determined from the predicted CL/F. This study is important towards individualization of dosing in the clinical setting and may increase the number of patients achieving the target concentration. This equation requires validation in an independent cohort of kidney transplant recipients.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures

References
-
- Bowman LJ, Brennan DC. The role of tacrolimus in renal transplantation. Expert Opin Pharmacother. 2008;9:635–43. - PubMed
-
- Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, Kuypers D, Le Meur Y, Marquet P, Oellerich M, Thervet E, Toenshoff B, Undre N, Weber LT, Westley IS, Mourad M. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31:139–52. - PubMed
-
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–53. - PubMed
-
- Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900–5. - PubMed
-
- Borobia AM, Romero I, Jimenez C, Gil F, Ramirez E, De Gracia R, Escuin F, Gonzalez E, Sansuan AJ. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit. 2009;31:436–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

