Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage
- PMID: 21672537
- PMCID: PMC3630073
- DOI: 10.1016/j.yexmp.2011.05.010
Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage
Abstract
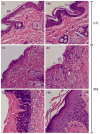
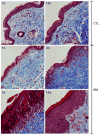
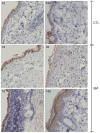
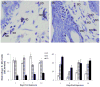
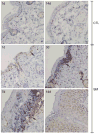
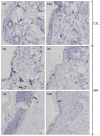
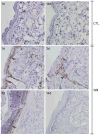
Sulfur mustard (SM, bis(2-chloroethyl)sulfide) is a bifunctional alkylating agent that causes dermal inflammation, edema and blistering. To investigate the pathogenesis of SM-induced injury, we used a vapor cup model which provides an occlusive environment in which SM is in constant contact with the skin. The dorsal skin of SKH-1 hairless mice was exposed to saturated SM vapor or air control. Histopathological changes, inflammatory markers and DNA damage were analyzed 1-14 days later. After 1 day, SM caused epidermal thinning, stratum corneum shedding, basal cell karyolysis, hemorrhage and macrophage and neutrophil accumulation in the dermis. Cleaved caspase-3 and phosphorylated histone 2A.X (phospho-H2A.X), markers of apoptosis and DNA damage, respectively, were increased whereas proliferating cell nuclear antigen (PCNA) was down-regulated after SM exposure. By 3 days, epithelial cell hypertrophy, edema, parakeratosis and loss of epidermal structures were noted. Enzymes generating pro-inflammatory mediators including myeloperoxidase and cyclooxygenase-2 were upregulated. After 7 days, keratin-10, a differentiation marker, was evident in the stratum corneum. This was associated with an underlying eschar, as neoepidermis began to migrate at the wound edges. Trichrome staining revealed increased collagen deposition in the dermis. PCNA expression in the epidermis was correlated with hyperplasia, hyperkeratosis, and parakeratosis. By 14 days, there was epidermal regeneration with extensive hyperplasia, and reduced expression of cleaved caspase-3, cyclooxygenase-2 and phospho-H2A.X. These findings are consistent with the pathophysiology of SM-induced skin injury in humans suggesting that the hairless mouse can be used to investigate the dermatoxicity of vesicants and the potential efficacy of countermeasures.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Alfredsson J, Puthalakath H, Martin H, Strasser A, Nilsson G. Proapoptotic Bcl-2 family member Bim is involved in the control of mast cell survival and is induced together with Bcl-XL upon IgE-receptor activation. Cell Death Differ. 2005;12:136–44. - PubMed
-
- Anderson DR, Mitcheltree LW, Brobst DE, Byers SL, Merz DF, Gold MB. A vapor exposure model for neonatal mice. Toxicol Mech Methods. 2002;12:59–70. - PubMed
-
- Bacchi CE, Gown AM. Detection of cell proliferation in tissue sections. Braz J Med Biol Res. 1993;26:677–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA132624/CA/NCI NIH HHS/United States
- CA132624/CA/NCI NIH HHS/United States
- AR055073/AR/NIAMS NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- CA093798/CA/NCI NIH HHS/United States
- R01 CA093798/CA/NCI NIH HHS/United States
- R01 GM034310/GM/NIGMS NIH HHS/United States
- U54 AR055073/AR/NIAMS NIH HHS/United States
- ES005022/ES/NIEHS NIH HHS/United States
- GM034310/GM/NIGMS NIH HHS/United States
- ES004738/ES/NIEHS NIH HHS/United States
- U54AR055073/AR/NIAMS NIH HHS/United States
- R01 ES004738/ES/NIEHS NIH HHS/United States
- R55 CA093798/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

