pH dependent molecular self-assembly of octaphosphonate porphyrin of nanoscale dimensions: nanosphere and nanorod aggregates
- PMID: 21673901
- PMCID: PMC3111612
- DOI: 10.3390/ijms12031464
pH dependent molecular self-assembly of octaphosphonate porphyrin of nanoscale dimensions: nanosphere and nanorod aggregates
Retraction in
-
Retraction: Bhosale, S.V., et al. pH Dependent Molecular Self-Assembly of Octaphosphonate Porphyrin of Nanoscale Dimensions: Nanosphere and Nanorod Aggregates. Int. J. Mol. Sci. 2011, 12, 1464-1473.Int J Mol Sci. 2021 Jan 29;22(3):1327. doi: 10.3390/ijms22031327. Int J Mol Sci. 2021. PMID: 33573104 Free PMC article.
Abstract
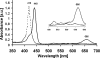
Self-assembled nanostructures of zwitterionic octaphosphanatoporphyrin 1, of either nanoparticles or nanorods, depending on small changes in the pH, is demonstrated based on the J-aggregates. Porphyrin 1 self-assembled into nanosphere aggregates with a diameter of about 70-80 nm in the pH range 5-7, and nanorod aggregates were observed at pH 8.5. Hydrogen bonding, π-π stacking and hydrophilic interactions play important roles in the formation of this nanostructure morphology. Nanostructures were characterized by UV/Vis absorbance, fluorescence, atomic force microscopy (AFM) and transmission electron microscopy (TEM). This interesting pH dependent self-assembly phenomenon could provide a basis for development of novel biomaterials.
Keywords: AFM; TEM; aggregation; molecular self-assembly; porphyrin.
Figures




References
-
- Inamura I, Uchida K. Association behavior of protoporphyrin IX in water and aqueous poly(N-vinyl pyrrolidone) solutions. Interactions between protoporphyrin IX and poly(N-vinyl pyrrolidone) Bull. Chem. Soc. Jpn. 1991;64:2005–2007.
-
- Fuhrhop JH, Bindig U, Siggel U. Micellar rods and vesicular tubules made of 14′″,16′″-diaminoporphyrins. J. Am. Chem. Soc. 1993;115:11036–11037.
-
- Fuhrhop J-H, Demoulin C, Boettcher C, Koening J, Siggel U. Chiral micellar porphyrin fibers with 2-aminoglycosamide head groups. J. Am. Chem. Soc. 1992;114:4159–4165.
-
- Porteu F, Palacin S, Ruandel-Teixier A, Barraud A. Supermolecular engineering at the air-water interface: Spatially controlled formation of heterodimers from amphiphilic porphyrins and porphyrazines through specific molecular recognition. J. Phys. Chem. 1991;95:7438–7441.
-
- Lei SB, Wang C, Yin SX, Wang HN, Xi F, Liu HW, Xu B, Wan LJ, Bai CL. Surface stabilized porphyrin and phthalocyanine two-dimensional network connected by hydrogen bonds. J. Phys. Chem. B. 2001;105:10838–10841.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

