Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases
- PMID: 21674054
- PMCID: PMC3107231
- DOI: 10.1371/journal.pone.0020758
Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases
Abstract
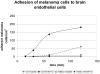
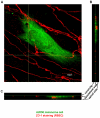
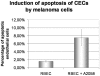
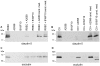
Malignant melanoma represents the third common cause of brain metastasis, having the highest propensity to metastasize to the brain of all primary neoplasms in adults. Since the central nervous system lacks a lymphatic system, the only possibility for melanoma cells to reach the brain is via the blood stream and the blood-brain barrier. Despite the great clinical importance, mechanisms of transmigration of melanoma cells through the blood-brain barrier are incompletely understood. In order to investigate this question we have used an in vitro experimental setup based on the culture of cerebral endothelial cells (CECs) and the A2058 and B16/F10 melanoma cell lines, respectively. Melanoma cells were able to adhere to confluent brain endothelial cells, a process followed by elimination of protrusions and transmigration from the luminal to the basolateral side of the endothelial monolayers. The transmigration process of certain cells was accelerated when they were able to use the routes preformed by previously transmigrated melanoma cells. After migrating through the endothelial monolayer several melanoma cells continued their movement beneath the endothelial cell layer. Melanoma cells coming in contact with brain endothelial cells disrupted the tight and adherens junctions of CECs and used (at least partially) the paracellular transmigration pathway. During this process melanoma cells produced and released large amounts of proteolytic enzymes, mainly gelatinolytic serine proteases, including seprase. The serine protease inhibitor Pefabloc® was able to decrease to 44-55% the number of melanoma cells migrating through CECs. Our results suggest that release of serine proteases by melanoma cells and disintegration of the interendothelial junctional complex are main steps in the formation of brain metastases in malignant melanoma.
Conflict of interest statement
Figures





References
-
- Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control. 2009;16:248–255. - PubMed
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Bauer HC, Traweger A, Zweimueller-Mayer J, Lehner C, Tempfer H, et al. New aspects of the molecular constituents of tissue barriers. J Neural Transm. 2011;118:7–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

