Site-specific chemical modification of a glycoprotein fragment expressed in yeast
- PMID: 21674341
- PMCID: PMC3141298
- DOI: 10.1007/978-1-61779-151-2_21
Site-specific chemical modification of a glycoprotein fragment expressed in yeast
Abstract
Site-specific modification of glycoproteins has wide application in both biochemical and biophysical studies. This method describes the conjugation of synthetic molecules to the N-terminus of a glycoprotein fragment, viz., human immunoglobulin G subclass 1 fragment crystallizable (IgG1 Fc), by native chemical ligation. The glycosylated IgG1 Fc is expressed in a glycosylation-deficient yeast strain. The N-terminal cysteine is generated by the endogenous yeast protease Kex2 in the yeast secretory pathway. The N-terminal cysteine is then conjugated with a biotin thioester to produce a biotinylated, glycosylated IgG1 Fc using native chemical ligation.
Figures
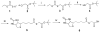




References
-
- Hackenberger CPR, Schwarzer D. Chemoselective ligation and modification strategies for peptides and proteins. Angew. Chem. Int. Ed. 2008;47:10030–10074. - PubMed
-
- Flavell RR, Muir TW. Expressed protein ligation (EPL) in the study of signal transduction, ion conduction, and chromatin biology. Acc. Chem. Res. 2009;42:107–116. - PubMed
-
- Xiao J, Burn A, Tolbert TJ. Increasing Solubility of Proteins and Peptides by Site-Specific Modification with Betaine. Bioconjugate Chem. 2008;19:1113–1118. - PubMed
-
- Kochendoerfer GG. Site-specific polymer modification of therapeutic proteins. Curr. Opin. Chem. Biol. 2005;9:555–560. - PubMed
-
- Tolbert TJ, Wong C-H. Intein-Mediated Synthesis of Proteins Containing Carbohydrates and Other Molecular Probes. J. Am. Chem. Soc. 2000;122:5421–5428.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

