Japanese macaque encephalomyelitis: a spontaneous multiple sclerosis-like disease in a nonhuman primate
- PMID: 21674589
- PMCID: PMC3170450
- DOI: 10.1002/ana.22449
Japanese macaque encephalomyelitis: a spontaneous multiple sclerosis-like disease in a nonhuman primate
Abstract
Objective: To describe Japanese macaque encephalomyelitis (JME), a spontaneous inflammatory demyelinating disease occurring in the Oregon National Primate Research Center's (ONPRC) colony of Japanese macaques (JMs, Macaca fuscata).
Methods: JMs with neurologic impairment were removed from the colony, evaluated, and treated with supportive care. Animals were humanely euthanized and their central nervous systems (CNSs) were examined.
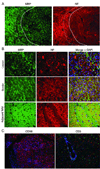
Results: ONPRC's JM colony was established in 1965 and no cases of JME occurred until 1986. Since 1986, 57 JMs spontaneously developed a disease characterized clinically by paresis of 1 or more limbs, ataxia, or ocular motor paresis. Most animals were humanely euthanized during their initial episode. Three recovered, later relapsed, and were then euthanized. There was no gender predilection and the median age for disease was 4 years. Magnetic resonance imaging of 8 cases of JME revealed multiple gadolinium-enhancing T(1) -weighted hyperintensities in the white matter of the cerebral hemispheres, brainstem, cerebellum, and cervical spinal cord. The CNS of monkeys with JME contained multifocal plaque-like demyelinated lesions of varying ages, including acute and chronic, active demyelinating lesions with macrophages and lymphocytic periventricular infiltrates, and chronic, inactive demyelinated lesions. A previously undescribed gamma-herpesvirus was cultured from acute JME white matter lesions. Cases of JME continue to affect 1% to 3% of the ONPRC colony per year.
Interpretation: JME is a unique spontaneous disease in a nonhuman primate that has similarities with multiple sclerosis (MS) and is associated with a novel simian herpesvirus. Elucidating the pathogenesis of JME may shed new light on MS and other human demyelinating diseases.
Copyright © 2011 American Neurological Association.
Figures







Comment in
-
On human disease and animal models.Ann Neurol. 2011 Sep;70(3):343-4. doi: 10.1002/ana.22552. Ann Neurol. 2011. PMID: 21905074 No abstract available.
References
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000 Sep 28;343(13):938–952. - PubMed
-
- Libbey J, Fujinami R. Potential Triggers of MS. In: Martin R, Lutterotti A, editors. Molecular Basis of Multiple Sclerosis. Springer Berlin / Heidelberg: 2010. pp. 21–42.
-
- Ferraro A, Cazzullo CL. Chronic experimental allergic encephalomyelitis in monkeys. J Neuropathol Exp Neurol. 1948 Jul;7(3):235–260. - PubMed
-
- Genain CP, Hauser SL. Creation of a model for multiple sclerosis in Callithrix jacchus marmosets. J Mol Med. 1997 Mar;75(3):187–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

