Direct plant tissue analysis and imprint imaging by desorption electrospray ionization mass spectrometry
- PMID: 21675752
- PMCID: PMC3137229
- DOI: 10.1021/ac201123t
Direct plant tissue analysis and imprint imaging by desorption electrospray ionization mass spectrometry
Abstract
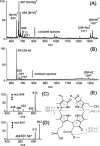
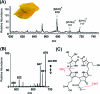
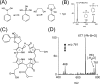
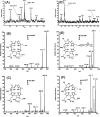
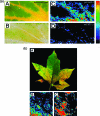
The ambient mass spectrometry technique, desorption electrospray ionization mass spectrometry (DESI-MS), is applied for the rapid identification and spatially resolved relative quantification of chlorophyll degradation products in complex senescent plant tissue matrixes. Polyfunctionalized nonfluorescent chlorophyll catabolites (NCCs), the "final" products of the chlorophyll degradation pathway, are detected directly from leaf tissues within seconds and structurally characterized by tandem mass spectrometry (MS/MS) and reactive-DESI experiments performed in situ. The sensitivity of DESI-MS analysis of these compounds from degreening leaves is enhanced by the introduction of an imprinting technique. Porous polytetrafluoroethylene (PTFE) is used as a substrate for imprinting the leaves, resulting in increased signal intensities compared with those obtained from direct leaf tissue analysis. This imprinting technique is used further to perform two-dimensional (2D) imaging mass spectrometry by DESI, producing well-resolved images of the spatial distribution of NCCs in senescent leaf tissues.
Figures
References
-
- Kräutler B.; Matile P. Acc. Chem. Res. 1999, 32, 35–43.
-
- Brown S. B.; Houghton J. D.; Hendry G. A. F. In Chlorophylls; Scheer H., Ed.; CRP-Press: Boca Raton, FL, 1991; pp 465–489.
-
- Kräutler B.; Jaun B.; Bortlik K.; Schellenberg M.; Matile P. Angew. Chem. Int. Ed. 1991, 30, 1315–1318.
-
- Kräutler B.; Hörtensteiner S. In Chlorophylls and Bacteriochlorophylls. Biochemistry, Biophysics, Functions and Applications, Advances in Photosynthesis and Respiration; Grimm B., Porra R., Rüdiger W., Scheer H., Eds.; Springer: Dordrecht, The Netherlands, 2006; Vol. 25; pp 237–260.
-
- Hörtensteiner S.; Kräutler B.. Biochim. Biophys. Acta 2010, DOI:10.1016/j.bbabio.2010.12.007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

