An alternative isomerohydrolase in the retinal Müller cells of a cone-dominant species
- PMID: 21676174
- PMCID: PMC3354629
- DOI: 10.1111/j.1742-4658.2011.08216.x
An alternative isomerohydrolase in the retinal Müller cells of a cone-dominant species
Abstract
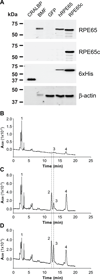
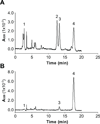
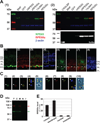
Cone photoreceptors have faster light responses than rods and a higher demand for 11-cis retinal (11cRAL), the chromophore of visual pigments. RPE65 is the isomerohydrolase in the retinal pigment epithelium (RPE) that converts all-trans retinyl ester to 11-cis retinol, a key step in the visual cycle for regenerating 11cRAL. Accumulating evidence suggests that cone-dominant species express an alternative isomerase, likely in retinal Müller cells, to meet the high demand for the chromophore by cones. In the present study, we describe the identification and characterization of a novel isomerohydrolase, RPE65c, from the cone-dominant zebrafish retina. RPE65c shares 78% amino acid sequence identity with RPE-specific zebrafish RPE65a (orthologue of human RPE65) and retains all of the known key residues for the enzymatic activity of RPE65. Similar to the other RPE-specific RPE65, RPE65c was present in both the membrane and cytosolic fractions, used all-trans retinyl ester as its substrate and required iron for its enzymatic activity. However, immunohistochemistry detected RPE65c in the inner retina, including Müller cells, but not in the RPE. Furthermore, double-immunostaining of dissociated retinal cells using antibodies for RPE65c and glutamine synthetase (a Müller cell marker), showed that RPE65c co-localized with the Müller cell marker. These results suggest that RPE65c is the alternative isomerohydrolase in the intra-retinal visual cycle, providing 11cRAL to cone photoreceptors in cone-dominant species. Identification of an alternative visual cycle will contribute to the understanding of the functional differences of rod and cone photoreceptors.
© 2011 The Authors Journal compilation © 2011 FEBS.
Figures



References
-
- Bridges CDB. The rhodopsin-porphyropsin system. In: Dartnall HJA, editor. Handbook of Sensory Physiology. Berlin: Springer; 1972. pp. 417–480.
-
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. - PubMed
-
- McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529. - PubMed
-
- Rando RR. The biochemistry of the visual cycle. Chem Rev. 2001;101:1881–1896. - PubMed
-
- Bernstein PS, Law WC, Rando RR. Biochemical characterization of the retinoid isomerase system of the eye. J Biol Chem. 1987;262:16848–16857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

