Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain
- PMID: 21676290
- PMCID: PMC5048912
- DOI: 10.1017/S1462399411001888
Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain
Abstract
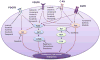
Glioblastoma multiforme, because of its invasive nature, can be considered a disease of the entire brain. Despite recent advances in surgery, radiotherapy and chemotherapy, current treatment regimens have only a marginal impact on patient survival. A crucial challenge is to deliver drugs effectively to invasive glioma cells residing in a sanctuary within the central nervous system. The blood-brain barrier (BBB) restricts the delivery of many small and large molecules into the brain. Drug delivery to the brain is further restricted by active efflux transporters present at the BBB. Current clinical assessment of drug delivery and hence efficacy is based on the measured drug levels in the bulk tumour mass that is usually removed by surgery. Mounting evidence suggests that the inevitable relapse and lethality of glioblastoma multiforme is due to a failure to effectively treat invasive glioma cells. These invasive cells hide in areas of the brain that are shielded by an intact BBB, where they continue to grow and give rise to the recurrent tumour. Effective delivery of chemotherapeutics to the invasive glioma cells is therefore critical, and long-term efficacy will depend on the ability of a molecularly targeted agent to penetrate an intact and functional BBB throughout the entire brain. This review highlights the various aspects of the BBB, and also the brain-tumour-cell barrier (a barrier due to expression of efflux transporters in tumour cells), that together can significantly influence drug response. It then discusses the challenge of glioma as a disease of the whole brain, which lends emphasis to the need to deliver drugs effectively across the BBB to reach both the central tumour and the invasive glioma cells.
Figures


References
-
- Altekruse SF, Kosary CL, Krapcho M, et al. National Cancer Institute; Bethesda, MD: 2010. SEER Cancer Statistics Review, 1975-2007. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010.
-
- CBTRUS. Central Brain Tumor Registry of the United States. Hinsdale, IL: 2010. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. website: www.cbtrus.org.
-
- Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010.
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Wen PY, Brandes AA. Treatment of recurrent high-grade gliomas. Curr Opin Neurol. 2009;22:657–664. - PubMed
Future Reading, Resources and Contacts
-
- Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. Reviews the latest developments in therapeutic options for glioma and highlights the current and future direction of clinical trials. - PMC - PubMed
-
- Lagas JS, Vlaming ML, Schinkel AH. Pharmacokinetic assessment of multiple ATP-binding cassette transporters: the power of combination knockout mice. Mol Interv. 2009;9(3):136–45. A review on the role of ABC transporters at the blood-brain barrier and the availability of newer research tools in the form of transgenic mice. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

