Missense mutation in APOC3 within the C-terminal lipid binding domain of human ApoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins: evidence that ApoC-III plays a major role in the formation of lipid precursors within the microsomal lumen
- PMID: 21676879
- PMCID: PMC3149367
- DOI: 10.1074/jbc.M110.203679
Missense mutation in APOC3 within the C-terminal lipid binding domain of human ApoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins: evidence that ApoC-III plays a major role in the formation of lipid precursors within the microsomal lumen
Abstract
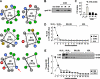
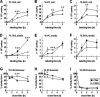
Hepatic assembly of triacylglycerol (TAG)-rich very low density lipoproteins (VLDL) is achieved through recruitment of bulk TAG (presumably in the form of lipid droplets within the microsomal lumen) into VLDL precursor containing apolipoprotein (apo) B-100. We determined protein/lipid components of lumenal lipid droplets (LLD) in cells expressing recombinant human apoC-III (C3wt) or a mutant form (K58E, C3KE) initially identified in humans that displayed hypotriglyceridemia. Although expression of C3wt markedly stimulated secretion of TAG and apoB-100 as VLDL(1), the K58E mutation (located at the C-terminal lipid binding domain) abolished the effect in transfected McA-RH7777 cells and in apoc3-null mice. Metabolic labeling studies revealed that accumulation of TAG in LLD was decreased (by 50%) in cells expressing C3KE. A Fat Western lipid protein overlay assay showed drastically reduced lipid binding of the mutant protein. Substituting Lys(58) with Arg demonstrated that the positive charge at position 58 is crucial for apoC-III binding to lipid and for promoting TAG secretion. On the other hand, substituting both Lys(58) and Lys(60) with Glu resulted in almost entire elimination of lipid binding and loss of function in promoting TAG secretion. Thus, the lipid binding domain of apoC-III plays a key role in the formation of LLD for hepatic VLDL assembly and secretion.
Figures

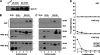


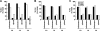

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

