The SUMO E3-ligase PIAS1 couples reactive oxygen species-dependent JNK activation to oxidative cell death
- PMID: 21676946
- PMCID: PMC3177572
- DOI: 10.1096/fj.11-186346
The SUMO E3-ligase PIAS1 couples reactive oxygen species-dependent JNK activation to oxidative cell death
Abstract
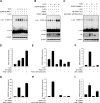
Human endometrial stromal cells (HESCs) exposed to reactive oxygen species (ROS) mount a hypersumoylation response in a c-Jun N-terminal kinase (JNK)-dependent manner. The mechanism that couples JNK signaling to the small ubiquitin-related modifier (SUMO) pathway and its functional consequences are not understood. We show that ROS-dependent JNK activation converges on the SUMO pathway via PIAS1 (protein inhibitor of activated STAT1). Unexpectedly, PIAS1 knockdown not only prevented ROS-dependent hypersumoylation but also enhanced JNK signaling in HESCs. Conversely, PIAS overexpression increased sumoylation of various substrates, including c-Jun, yet inhibited basal and ROS-dependent JNK activity independently of its SUMO ligase function. Expression profiling demonstrated that PIAS1 knockdown enhances and profoundly modifies the transcriptional response to oxidative stress signals. Using a cutoff of 2-fold change or more, a total of 250 ROS-sensitive genes were identified, 97 of which were not dependent on PIAS1. PIAS1 knockdown abolished the regulation of 43 genes but also sensitized 110 other genes to ROS. Importantly, PIAS1 silencing was obligatory for the induction of several cellular defense genes in response to oxidative stress. In agreement, PIAS1 knockdown attenuated ROS-dependent caspase-3/7 activation and subsequent apoptosis. Thus, PIAS1 determines the level of JNK activity in HESCs, couples ROS signaling to the SUMO pathway, and promotes oxidative cell death.
Figures
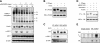

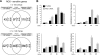
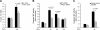

References
-
- Cheng J., Perkins N. D., Yeh E. T. (2005) Differential regulation of c-Jun-dependent transcription by SUMO-specific proteases. J. Biol. Chem. 280, 14492–14498 - PubMed
-
- Gellersen B., Brosens I. A., Brosens J. J. (2007) Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 25, 445–453 - PubMed
-
- Brosens J. J., Gellersen B. (2006) Death or survival–progesterone-dependent cell fate decisions in the human endometrial stromal. J. Mol. Endocrinol. 36, 389–398 - PubMed
-
- Gellersen B., Brosens J. (2003) Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J. Endocrinol. 178, 357–372 - PubMed
-
- Brosens J. J., Parker M. G., McEnroe A., Pijnenborg R., Brosens I. A. (2009) A role for menstruation in preconditioning the uterus for successful pregnancy. Am. J. Obstet. Gynecol. 200, 615.e1–615.e6 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

