Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients
- PMID: 21677143
- PMCID: PMC3131454
- DOI: 10.4049/jimmunol.1004195
Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients
Abstract
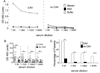
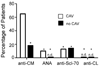
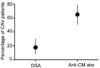
Chronic allograft vasculopathy (CAV) contributes to heart transplant failure, yet its pathogenesis is incompletely understood. Although cellular and humoral alloimmunity are accepted pathogenic mediators, animal models suggest that T cells and Abs reactive to graft-expressed autoantigens, including cardiac myosin (CM), could participate. To test the relationship between CAV and anti-CM autoimmunity in humans, we performed a cross-sectional study of 72 heart transplant recipients: 40 with CAV and 32 without. Sera from 65% of patients with CAV contained anti-CM Abs, whereas <10% contained Abs to other autoantigens (p < 0.05), and only 18% contained anti-HLA Abs (p < 0.05 versus anti-CM). In contrast, 13% of sera from patients without CAV contained anti-CM Abs (p < 0.05; odds ratio [OR], associating CAV with anti-CM Ab = 13, 95% confidence interval [CI] 3.79-44.6). Multivariable analysis confirmed the association to be independent of time posttransplant and the presence of anti-HLA Abs (OR = 28, 95% CI 5.77-133.56). PBMCs from patients with CAV responded more frequently to, and to a broader array of, CM-derived peptides than those without CAV (p = 0.01). Detection of either CM-peptide-reactive T cells or anti-CM Abs was highly and independently indicative of CAV (OR = 45, 95% CI 4.04-500.69). Our data suggest detection of anti-CM immunity could be used as a biomarker for outcome in heart transplantation recipients and support the need for further studies to assess whether anti-CM immunity is a pathogenic mediator of CAV.
Figures




References
-
- Johnston DR, Sayegh MH, Madsen JC. Overcoming cardiac allograft vasculopathy (CAV) by inducing tolerance. Front Biosci. 2002;7:e116–e118. - PubMed
-
- Mehra MR. Contemporary concepts in prevention and treatment of cardiac allograft vasculopathy. Am J Transplant. 2006;6:1248–1256. - PubMed
-
- Chen Y, Demir Y, Valujskikh A, Heeger PS. The male minor transplantation antigen preferentially activates recipient CD4+ T cells through the indirect presentation pathway in vivo. J Immunol. 2003;171:6510–6518. - PubMed
-
- Sahara H, Shoji T, Ng CY, Weiss MJ, Muniappan A, Guenther DA, Houser SL, Pujara AC, Sayre JK, Wain JC, Sachs DH, Madsen JC, Allan JS. The role of indirect recognition of MHC class I and II allopeptides in a fully mismatched miniature swine model of lung transplantation. Transplant Proc. 2006;38:3256–3258. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

