Cerebellar-dependent learning in larval zebrafish
- PMID: 21677154
- PMCID: PMC6622926
- DOI: 10.1523/JNEUROSCI.6565-10.2011
Cerebellar-dependent learning in larval zebrafish
Abstract
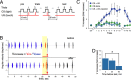
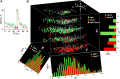
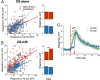
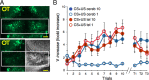
Understanding how neuronal network activity contributes to memory formation is challenged by the complexity of most brain circuits and the restricted ability to monitor the activity of neuronal populations in vivo. The developing zebrafish (Danio rerio) is an animal model that circumvents these problems, because zebrafish larvae possess a rich behavioral repertoire and an accessible brain. Here, we developed a classical conditioning paradigm in which 6- to 8-d-old larvae develop an enhanced motor response to a visual stimulus (conditioned stimulus, CS) when it is paired with touch (unconditioned stimulus, US). Using in vivo calcium imaging we demonstrate that CS and US activate different subsets of neurons in the cerebellum; their activity, modulated by learning two-photon laser ablation, revealed that the cerebellum is involved in acquisition and extinction, but not the retention, of this memory.
Figures
References
-
- Bae YK, Kani S, Shimizu T, Tanabe K, Nojima H, Kimura Y, Higashijima S, Hibi M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev Biol. 2009;330:406–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
