Microwave assisted synthesis of some new fused 1,2,4-triazines bearing thiophene moieties with expected pharmacological activity
- PMID: 21677606
- PMCID: PMC6264204
- DOI: 10.3390/molecules16064937
Microwave assisted synthesis of some new fused 1,2,4-triazines bearing thiophene moieties with expected pharmacological activity
Abstract
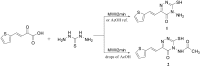
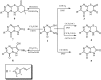
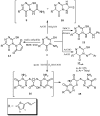
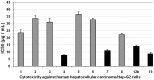
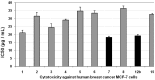
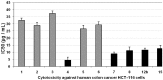
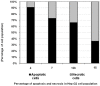
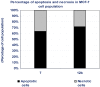
Rapid and efficient solvent-free synthesis of 4-amino-3-mercapto-6-[2-(2-thienyl)vinyl]-1,2,4-triazin-5(4H)-one 1 under microwave irradiation is described. Some new fused heterobicyclic nitrogen systems such as 1,2,4-triazino[3,4-b][1,3,4]thiadiazinones, 1,3,4-thiadiazolo[2,3-c][1,2,4]triazinone and pyrazolo[5,1-c]-[1,2,4]triazine-7-carbonitrile, have been synthesized by treatment of 1 with bifunctional oxygen and halogen compounds, CS₂/KOH and malononitrile via heterocyclization reactions, in addition to some uncondensed triazines. Structures of the products have been deduced from their elemental analysis and spectral data (IR, ¹H-NMR, ¹³C-NMR). Select new synthesized compounds were screened as anticancer agents, with some showing activity as cytotoxic agents against different cancer cell lines.
Figures
References
-
- Chow W.S., Chan T.H. Microwave-assisted solvent-free N-arylation of imidazole and pyrazole. Tetrahedron Lett. 2009;50:1286–1289. doi: 10.1016/j.tetlet.2008.12.119. - DOI
-
- Abdul Rauf S.S., Gangal S. Microwave assisted efficient one-pot synthesis of 3,5,6-trisubstituted-1,2,4-triazines from fatty acid hydrazides under solvent-free conditions and their antimicrobial activity. ARKIVOC. 2007;xvi:137–147.
-
- Shipe W.D., Wolkenberg S.E., Lindsley C.W. Accelerating lead development by microwave-enhanced medicinal chemistry. Drug Discov. Today Tech. 2005;2:155–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

