Latent TGF-β structure and activation
- PMID: 21677751
- PMCID: PMC4717672
- DOI: 10.1038/nature10152
Latent TGF-β structure and activation
Abstract
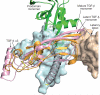
Transforming growth factor (TGF)-β is stored in the extracellular matrix as a latent complex with its prodomain. Activation of TGF-β1 requires the binding of α(v) integrin to an RGD sequence in the prodomain and exertion of force on this domain, which is held in the extracellular matrix by latent TGF-β binding proteins. Crystals of dimeric porcine proTGF-β1 reveal a ring-shaped complex, a novel fold for the prodomain, and show how the prodomain shields the growth factor from recognition by receptors and alters its conformation. Complex formation between α(v)β(6) integrin and the prodomain is insufficient for TGF-β1 release. Force-dependent activation requires unfastening of a 'straitjacket' that encircles each growth-factor monomer at a position that can be locked by a disulphide bond. Sequences of all 33 TGF-β family members indicate a similar prodomain fold. The structure provides insights into the regulation of a family of growth and differentiation factors of fundamental importance in morphogenesis and homeostasis.
Figures


References
-
- Wu MY, Hill CS. TGF-β superfamily signaling in embryonic development and homeostasis. Dev. Cell. 2009;16:329–343. - PubMed
-
- Derynck R, Miyazono K. In: The TGF-β Family. Derynck R, Miyazono K, editors. Cold Spring Harbor Laboratory Press; 2008. pp. 29–43. Ch. 2.
-
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000;342:1350–1358. - PubMed
-
- Gray AM, Mason AJ. Requirement for activin A and transforming growth factor-β 1 pro-regions in homodimer assembly. Science. 1990;247:1328–1330. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

