Trans-catheter closure of atrial septal defect: Balloon sizing or no Balloon sizing - single centre experience
- PMID: 21677801
- PMCID: PMC3104527
- DOI: 10.4103/0974-2069.79619
Trans-catheter closure of atrial septal defect: Balloon sizing or no Balloon sizing - single centre experience
Abstract
Background: Selecting the device size using a sizing balloon could oversize the ostium secundum atrial septal defect (OSASD) with floppy margins and at times may lead to complications. Identifying the firm margins using trans-esophageal echocardiography (TEE) and selecting appropriate-sized device optimizes ASD device closure. This retrospective study was undertaken to document the safety and feasibility of device closure without balloon sizing the defect.
Methods: Sixty-one consecutive patients who underwent trans-catheter closure of OSASD guided by balloon sizing of the defect and intra procedural fluoroscopy (group I) and 67 consecutive patients in whom TEE was used for defect sizing and as intraprocedural imaging during device deployment (group II) were compared. The procedural success rate, device characteristics, and complications were compared between the two groups.
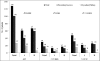
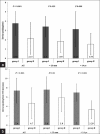
Results: The procedure was successful in 79.7 % patients. The success rate in group II (60 of 67, 89.6%) was significantly higher than in group I (41 of 61, 67.2 %) (P = 0.002). Mean upsizing of ASD device was significantly lower in group II (P < 0.001). TEE also provided better success rate with smaller device in subjects with large ASD (>25 mm) and in those who were younger than 14 years of age. There were four cases of device embolization (two in each group); of which one died in group II despite successful surgical retrieval.
Conclusion: Balloon sizing may not be essential for successful ASD device closure. TEE-guided sizing of ASD and device deployment provides better success rate with relatively smaller sized device.
Keywords: Atrial septal defect; balloon sizing; trans-catheter closure; trans-esophageal echocardiography.
Conflict of interest statement
Figures
References
-
- Berger F, Ewert P, Bjornstad PG, Dahnert I, Krings G, Brilla- Austenat I, et al. Transcatheter closure as standard treatment for most interatrial defects: Experience in 200 patients treated with the Amplatzer septal occluder. Cardiol Young. 1999;9:468–73. - PubMed
-
- Chessa M, Carminati M, Butrera G, Bini RM, Drago M, Rosti L, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002;39:1061–5. - PubMed
-
- Du ZD, Hijazi ZM, Kleinman CS, Silverman NH. Amplatzer investigators.Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: Results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1936–44. - PubMed
-
- Thanopoulos BD, Laskari CV, Tsaousis GS, Zarayelyan A, Vekiou A, Papadopoulos GS. Closure of atrial septal defects with the Amplatzer occlusion device: Preliminary results. J Am Coll Cardiol. 1998;31:1110–6. - PubMed
-
- Wang JK, Tsai SK, Wu MH, Lue HC. Short-and intermediate-term results of transcatheter closure of atrial septal defect with the Amplatzer septal occluder. Am Heart J. 2004;148:511–7. - PubMed
LinkOut - more resources
Full Text Sources
Medical