Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy
- PMID: 21677874
- PMCID: PMC3114244
- DOI: 10.1593/neo.101590
Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy
Abstract
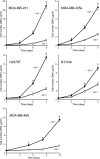
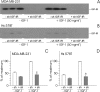
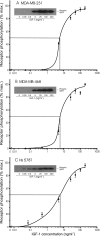
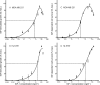
Triple-negative breast cancers have a poor prognosis and are not amenable to endocrine- or HER2-targeted therapies. The prevailing view is that targeting the insulin-like growth factor (IGF) signal transduction pathway will not be beneficial for triple-negative breast cancers because their growth is not IGF-responsive. The present study investigates the importance of IGFs in the proliferation and survival of triple-negative breast cancer cells. Estrogen and progesterone receptors, HER2, type I IGF, and insulin receptors were measured by Western transfer analysis. The effects of IGF-1 on proliferation were assessed by DNA quantitation and on cell survival by poly (ADP-ribose) polymerase cleavage. The effect of IGF-1 on phosphorylation of the IGF receptors, Akt and mitogen-activated protein kinase, was measured by Western transfer analysis. Seven cell lines were identified as models of triple-negative breast cancer and shown to express IGF receptors at levels similar to those present in estrogen-responsive cell lines known to respond to IGFs. IGF-1 increased the proliferation and cell survival of all triple-negative cell lines. Proliferation was attenuated after reduction of type I IGF receptor expression. Cells that express higher levels of receptor were more sensitive to subnanomolar IGF-1 concentrations, but the magnitude of the effects was not correlated simply with the absolute amount or phosphorylation of the IGF receptors, Akt or mitogen-activated protein kinase. These results show that IGFs stimulate cell proliferation and promote cell survival in triple-negative breast cancer cells and warrant investigation of the IGF signal transduction pathway as a therapeutic target for the treatment of triple-negative breast cancer.
Figures



References
-
- Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14(24):8010–8018. - PubMed
-
- Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52(1):108–118. - PubMed
-
- Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–2581. - PubMed
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109(9):1721–1728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
