Adjuvant progestagens for endometrial cancer
- PMID: 21678331
- PMCID: PMC4238061
- DOI: 10.1002/14651858.CD001040.pub2
Adjuvant progestagens for endometrial cancer
Abstract
Background: Endometrial cancer is the most common genital tract carcinoma among women in developed countries, with most women presenting with stage 1 disease. Adjuvant progestagen therapy has been advocated following primary surgery to reduce the risk of recurrence of disease.
Objectives: To evaluate the effectiveness and safety of adjuvant progestagen therapy for the treatment of endometrial cancer.
Search strategy: We searched the Cochrane Gynaecological Cancer Group Trials Specilaised Register, Cochrane Central Register of Controlled Trials (CENTRAL) Issue 2, 2009. MEDLINE and EMBASE up to April 2009.
Selection criteria: Randomised controlled trials (RCTs) of progestagen therapy in women who have had surgery for endometrial cancer.
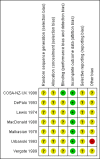
Data collection and analysis: Two review authors independently abstracted data and assessed risk of bias. Risk ratios (RRs) comparing survival in women who did and did not receive progestagen were pooled in random effects meta-analyses. .
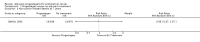
Main results: Seven trials assessing 4556 women were identified. Three trials included women with stage one disease only, whereas four included women with more advanced disease. Meta-analysis of four trials showed that there was no significant difference in the risk of death at five years between adjuvant progestagen therapy and no further treatment (RR = 1.00, 95% CI 0.85 to 1.18). This conclusion is also robust to single trial analyses at 4 and 7 years and in one trial across all points in time using a hazard ratio (HR). There was also no significant difference between progestagen therapy and control in terms of the risk of death from endometrial cancer, cardiovascular disease and intercurrent disease. Relapse of disease appeared to be reduced by progestagen therapy in one trial (HR = 0.71, 95% CI 0.52 to 0.97 and 5 year RR = 0.74, 95% CI 0.58 to 0.96), but there was no evidence of a difference in disease recurrence in another trial at 7 years (RR = 1.34, 95% CI 0.79 to 2.27).
Authors' conclusions: There is no evidence to support the use of adjuvant progestagen therapy in the primary treatment of endometrial cancer.
Conflict of interest statement
None known
Figures
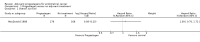



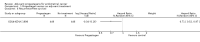

Update of
-
Progestagens for endometrial cancer.Cochrane Database Syst Rev. 2000;(2):CD001040. doi: 10.1002/14651858.CD001040. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2011 Jun 15;(6):CD001040. doi: 10.1002/14651858.CD001040.pub2. PMID: 10796737 Updated.
References
References to studies included in this review
COSA‐NZ‐UK 1998 {published data only}
-
- COSA‐NZ‐UK Endometrial Cancer Groups. Adjuvant medroxyprogesterone acetate in high‐risk endometrial cancer. International Journal of Gynecological Cancer 1998;8:387‐91.
DePalo 1993 {published data only}
-
- DePalo G, Mangioni C, Periti P, Vecchio M, Marubini E. Treatment of FIGO (1971) stage 1 endometrial carcinoma with intensive surgery, radiotherapy and hormonotherapy according to pathological prognostic groups. Long term results of a randomised multicentre trial. European Journal of Cancer 1993;29a:1133‐40. - PubMed
Lewis 1974 {published data only}
-
- Lewis GC, Slack NH, Mortel R, Bross I. Adjuvant progestagen therapy in the definitive treatment of endometrial cancer. Gynecologic Oncology 1974;2:368‐76. - PubMed
MacDonald 1988 {published data only}
-
- MacDonald RR, Thorogood J, Mason MK. A randomised trial of progestagens in the primary treatment of endometrial carcinoma. British Journal of Obstetrics and Gynaecology 1988;95:166‐74. - PubMed
Malkasian 1978 {published data only}
-
- Malkasian G, Decker D. Aduvant progesterone therapy for stage 1 endometrial cancer. International Journal of Gynaecology and Obstetrics 1978;16:48‐9. - PubMed
Urbanski 1993 {published data only}
-
- Urbanski K, Karolewski K, Kojs Z, Klimek M, Dyba T. Adjuvant progestagen therapy improves survival in patients with endometrial cancer after hysterectomy. results of one‐institutional prospective clinical trial. European Journal of Gynaecologic Oncology 1993;14 suppl:98‐104. - PubMed
Vergote 1989 {published data only}
-
- Vergote I, Kjorstad K, Abeler V, Kolstad P. A randomised trial of adjuvant progestagens in early endometrial cancer. Cancer 1989;64:1011‐6. - PubMed
Additional references
Baekerlandt 2009
-
- Baekelandt MM, Castiglione M, on behalf of the ESMO Guidelines Working Group. Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow‐up [Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow‐up]. Annals of Oncology. 2009;20(supplement 4):29‐31. - PubMed
Cancer Research UK 2010
-
- Uterus (womb) cancer ‐ UK incidence statistics. http://info.cancerresearchuk.org/cancerstats/types/uterus/incidence/inde.... 2010.
CTCAE 2006
-
- CTCAE. Common Terminology Criteria for Adverse Events. (http://ctep.cancer.gov/forms/CTCAEv3.pdf) 9th August 2006; Vol. v3.0 (CTCAE).
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). London: BMJ Publication Group, 2001.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177‐188. - PubMed
EUROCARE 2003
-
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, Grosclaude P, Hédelin G, Matsuda T, Møller H, Möller T, Verdecchia A, Capocaccia R, Gatta G, Micheli A, Santaquilani M, Roazzi P, Lisi D, and the EUROCARE Working Group. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14 (Supplement 5):v61‐v118. - PubMed
GLOBOCAN 2008
-
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No. 5, version 2.0. IARCPress, Lyon 2004.
Higgins 2003
Kauppila 1984
-
- Kauppila A. Progestin therapy of endometrial, breast and ovarian carcinoma. A review of clinical observations.. Acta Obstet Gynecol Scand. 1984;63(5):441‐50. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Pecorelli 2009
-
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. International Journal of Gynecology and Obstetrics 2009;105(2):103‐4. - PubMed
SEER 2011
-
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. Surveillance Epidemiology and End Results Cancer Statistics Review, 1975‐2008. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

