Cardioprotective interventions for cancer patients receiving anthracyclines
- PMID: 21678342
- PMCID: PMC6457676
- DOI: 10.1002/14651858.CD003917.pub4
Cardioprotective interventions for cancer patients receiving anthracyclines
Abstract
Background: Anthracyclines are among the most effective chemotherapeutic agents in the treatment of numerous malignancies. Unfortunately, their use is limited by a dose-dependent cardiotoxicity. In an effort to prevent this cardiotoxicity, different cardioprotective agents have been studied.
Objectives: The objective of this review was to assess the efficacy of different cardioprotective agents in preventing heart damage in cancer patients treated with anthracyclines.
Search strategy: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 10), MEDLINE (1966 to November 2010) and EMBASE (1980 to November 2010) databases. In addition, we handsearched reference lists, conference proceedings of the International Society of Paediatric Oncology (SIOP) and American Society of Clinical Oncology (ASCO) meetings (1998 to 2010) and ongoing trials registers.
Selection criteria: Randomised controlled trials (RCTs) in which any cardioprotective agent was compared to no additional therapy or placebo in cancer patients (children and adults) receiving anthracyclines.
Data collection and analysis: Two review authors independently performed the study selection, risk of bias assessment and data extraction including adverse effects.
Main results: We identified RCTs for the eight cardioprotective agents N-acetylcysteine, phenethylamines, coenzyme Q10, a combination of vitamins E and C and N-acetylcysteine, L-carnitine, carvedilol, amifostine and dexrazoxane (mostly for adults with advanced breast cancer). All studies had methodological limitations and for the first seven agents there were too few studies to allow pooling of results. None of the individual studies showed a cardioprotective effect. The 10 included studies on dexrazoxane enrolled 1619 patients. The meta-analysis for dexrazoxane showed a statistically significant benefit in favour of dexrazoxane for the occurrence of heart failure (risk ratio (RR) 0.29, 95% CI 0.20 to 0.41). No evidence was found for a difference in response rate or survival between the dexrazoxane and control groups. The results for adverse effects were ambiguous. No significant difference in the occurrence of secondary malignancies was identified.
Authors' conclusions: No definitive conclusions can be made about the efficacy of cardioprotective agents for which pooling of results was impossible. Dexrazoxane prevents heart damage and no evidence for a difference in response rate or survival between the dexrazoxane and control groups was identified. The evidence available did not allow us to reach any definite conclusions about adverse effects. We conclude that if the risk of cardiac damage is expected to be high, it might be justified to use dexrazoxane in patients with cancer treated with anthracyclines. However, clinicians should weigh the cardioprotective effect of dexrazoxane against the possible risk of adverse effects for each individual patient.
Conflict of interest statement
None known
Figures



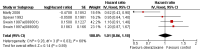
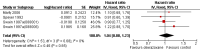

























Update of
-
Cardioprotective interventions for cancer patients receiving anthracyclines.Cochrane Database Syst Rev. 2008 Apr 16;(2):CD003917. doi: 10.1002/14651858.CD003917.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2011 Jun 15;(6):CD003917. doi: 10.1002/14651858.CD003917.pub4. PMID: 18425895 Updated.
References
References to studies included in this review
Galetta 2005 {published data only}
-
- Galetta F, Franzoni F, Cervetti G, Cecconi N, Carpi A, Petrini M, et al. Effect of epirubicin‐based chemotherapy and dexrazoxane supplementation on QT dispersion in non‐Hodgkin lymphoma patients. Biomedicine & Pharmacotherapy 2005;59:541‐4. - PubMed
Gallegos‐Castorena 2007 {published data only}
-
- Gallegos‐Castorena S, Martínez‐Avalos A, Mohar‐Betancourt A, Guerrero‐Avendaño G, Zapata‐Tarrés M, Medina‐Sansón A. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatric Hematology and Oncology 2007;24:403‐8. - PubMed
Iarussi 1994 {published data only}
-
- Iarussi D, Auricchio U, Agretto A, Murano A, Giuliano M, Casale F, et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non‐hodgkin lymphoma. Molecular Aspects of Medicine 1994;15 Suppl:207‐12. - PubMed
Kalay 2006 {published data only}
-
- Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline‐induced cardiomyopathy. Journal of the American College of Cardiology 2006;48:2258‐62. - PubMed
Kraft 1990 {published data only}
-
- Kraft J, Grille W, Appelt M, Hossfeld DK, Eichelbaum M, Koslowski B, et al. Effects of verapamil on anthracycline‐induced cardiomyopathy: preliminary results of a prospective multicenter trial. Haematology and Blood Transfusion 1990;33:566‐70. - PubMed
Lipshultz 2004 {published data only}
-
- Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high‐risk acute lymphoblastic leukemia treated with dexrazoxane. Journal of Clinical Oncology 2008;26:1106‐11. - PubMed
-
- Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin‐treated children with acute lymphoblastic leukemia. The New England Journal of Medicine 2004;351:145‐53. - PubMed
-
- Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin‐treated children with high‐risk acute lymphoblastic leukaemia: long‐term follow‐up of a prospective, randomised, multicentre trial. Lancet Oncology 2010;11:950‐61. - PMC - PubMed
Lopez 1998 {published data only}
-
- Lopez M, Vici P, Lauro L, Conti F, Paoletti G, Ferraironi A, et al. Randomized prospective clinical trial of high‐dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. Journal of Clinical Oncology 1998;16:86‐92. - PubMed
Marty 2006 {published data only}
-
- Marty M, Espie M, Llombart A, Monnier A, Rapoport BL, Stahalova V. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline‐based chemotherapy. Annals of Oncology 2006;17:614‐22. - PubMed
Milei 1987 {published data only}
-
- Milei J, Marantz A, Ale J, Vazquez A, Buceta JE. Prevention of adriamycin‐induced cardiotoxicity by prenylamine: a pilot double blind study. Cancer Drug Delivery 1987;4:129‐36. - PubMed
Myers 1983 {published data only}
-
- Myers C, Bonow R, Palmeri S, Jenkins J, Corden B, Locker G, et al. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N‐acetylcysteine. Seminars in Oncology 1983;10 Suppl 1(1):53‐5. - PubMed
Schwartz 2009 {published data only}
Speyer 1992 {published data only}
-
- Speyer JL, Green MD, Zeleniuch‐Jacquotte A, Wernz JC, Rey M, Sanger J, et al. ICRF‐187 permits longer treatment with doxorubicin in women with breast cancer. Journal of Clinical Oncology 1992;10:117‐27. - PubMed
Swain 1997a(088001) {published data only}
-
- Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin‐containing therapy in advanced breast cancer. Journal of Clinical Oncology 1997;15:1318‐32. - PubMed
Swain 1997a(088006) {published data only}
-
- Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin‐containing therapy in advanced breast cancer. Journal of Clinical Oncology 1997;15:1318‐32. - PubMed
Venturini 1996 {published data only}
-
- Venturini M, Michelotti A, Mastro L, Gallo L, Carnino F, Garrone O, et al. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. Journal of Clinical Oncology 1996;14:3112‐20. - PubMed
Wagdi 1995 {published data only}
-
- Wagdi PH, Rouvinez G, Fluri M, Aeschbacher B, Thoni A, Schefer H, et al. Cardioprotection during chemo‐and radiotherapy of malignant diseases ‐ an echocardiographic pilot study [Kardioprotektion bei chemo‐und radiotherapie fur maligne erkrankungen ‐ eine echokardiographische pilotstudie]. Schweizerische Rundschau fur Medizin (Praxis) 1995;84:1220‐3. - PubMed
Waldner 2006 {published data only}
Wexler 1996 {published data only}
-
- Wexler LH, Andrich MP, Venzon D, Berg SL, Weaver‐McClure L, Chen CC, et al. Randomized trial of the cardioprotective agent ICRF‐187 in pediatric sarcoma patients treated with doxorubicin. Journal of Clinical Oncology 1996;14:362‐72. - PubMed
References to studies excluded from this review
Bates 1997 {published data only}
-
- Bates M, Lieu D, Zagari M, Spiers A, Williamson T. A pharmacoeconomic evaluation of the use of dexrazoxane in preventing anthracycline‐induced cardiotoxicity in patients with stage IIIB or IV metastatic breast cancer. Clinical Therapeutics 1997;19:167‐84. - PubMed
Batist 1985 {published data only}
-
- Batist G, Norton J, Katki AG, Wagman L, Ferrans VJ, Maher M, et al. Cardiac and red blood cell glutathione peroxidase: results of a prospective randomized trial in patients on total parenteral nutrition. Cancer Research 1985;45:5900‐3. - PubMed
Cardinale 2006 {published data only}
-
- Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation 2006;114:2474‐81. - PubMed
Cascinu 1995 {published data only}
-
- Cascinu S, Cordella L, Ferro E, Fronzoni M, Catalano G. Neuroprotective effect of reduced glutathione on cisplatin‐based chemotherapy in advanced gastric cancer: a randomized double‐blind placebo‐controlled trial. Journal of Clinical Oncology 1995;13:26‐32. - PubMed
Judy 1984 {published data only}
-
- Judy WV, Hall JH, Dugan W, Toth PD, Folkers K. Coenzyme Q10 reduction of adriamycin cardiotoxicity. In: Folkers K, Yamamura Y editor(s). Biomedical and clinical aspects of coenzyme Q. Vol. 4, Amsterdam: Elsevier / North‐Holland Biomedical Press, 1984:231‐41.
Massida 1997 {published data only}
-
- Massidda B, Fenu MA, Ionta MT, Tronci M, Foddi MR, Montaldo C, et al. Early detection of the anthracycline‐induced cardiotoxicity. A non‐invasive haemodynamic study. Anticancer Research 1997;17:663‐8. - PubMed
Michelotti 2000 {published data only}
-
- Michelotti A, Venturini M, Tibaldi C, Bengala C, Gallo L, Carnino F, et al. Single agent epirubicin as first line chemotherapy for metastatic breast cancer patients. Breast Cancer Research and Treatment 2000;59:133‐9. - PubMed
Moghrabi 2007 {published data only}
Nakamae 2005 {published data only}
-
- Nakamae H, Tsumura K, Terada Y, Nakane T, Nakamae M, Ohta K, et al. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 2005;104:2492‐8. - PubMed
Neto 2006 {published data only}
-
- Neto RPDM, Petrilli AS, Silva CMC, Campos Filho O, Oporto VM, Gomes LDFG, et al. Left ventricular systolic function assessed by echocardiography in children and adolescents with osteosarcoma treated with doxorubicin alone or in combination with dexrazoxane. Arquivos Brasileiros de Cardiologia 2006;87:699‐706. - PubMed
Paiva 2005 {published data only}
-
- Paiva MG, Petrilli AS, Moises VA, Macedo CR, Tanaka C, Campos O. Cardioprotective effect of dexrazoxane during treatment with doxorubicin: a study using low‐dose dobutamine stress echocardiography. Pediatric Blood and Cancer 2005;45:902‐8. - PubMed
Piccinini 1987 {published data only}
-
- Piccinini F, Monti E, Paracchini L. Protective effect of trimetazidine on the cardiotoxicity of doxorubicin. Concours Medical 1987;109:3479‐83.
Rosenfeld 1992 {published data only}
-
- Rosenfeld CS, Weisberg SR, York RM, Jones SE, Spicer DV, Khojasteh A, et al. Prevention of adriamycin cardiomyopathy with dexrazoxane (ADR‐529, ICRF‐187). Proceedings of ASCO 1992;11:62.
Speyer 1988 {published data only}
-
- Speyer JL, Green MD, Kramer E, Rey M, Sanger J, Ward C, et al. Protective effect of the bispiperazinedione ICRF‐187 against doxorubicin‐induced cardiac toxicity in women with advanced breast cancer. The New England Journal of Medicine 1988;319:745‐52. - PubMed
Speyer 1990 {published data only}
-
- Speyer JL, Green MD, Sanger J, Zeleniuch‐Jacquotte A, Kramer E, Rey M, et al. A prospective randomized trial of ICRF‐187 for prevention of cumulative doxorubicin‐induced cardiac toxicity in women with breast cancer. Cancer Treatment Reviews 1990;17:161‐3. - PubMed
Swain 1997b {published data only}
-
- Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin‐containing therapy. Journal of Clinical Oncology 1997;15:1333‐40. - PubMed
Tallarico 2003 {published data only}
-
- Tallarico D, Rizzo V, Maio F, Petretto F, Bianco G, Placanica G, et al. Myocardial cytoprotection by trimetazidine against anthracycline‐induced cardiotoxicity in anticancer chemotherapy. Angiology 2003;54:219‐27. - PubMed
Tebbi 2007 {published data only}
-
- Tebbi CK, London WB, Friedman F, Villaluna D, Alarcon PA, Constine LS, et al. Dexrazoxane‐associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. Journal of Clinical Oncology 2007;25:493‐500. - PubMed
Ten Bokkel 1990 {published data only}
-
- Bokkel‐Huinink WW, Rodenhuis S, Schreuder JE, Dubbelman R, Bierhorst F, Tinteren H, et al. ICRF 187 protects against doxorubicin induced cardiomyopathy. Annals of Oncology 1990;1:106.
Tonkin 1996 {published data only}
-
- Tonkin K, Bates M, Lieu D, Arundell E, Williamson TY, Zagari M. Dexrazoxane cardioprotection for patients receiving FAC chemotherapy: a pharmacoeconomic evaluation. Annals of Cancer Control Research 1996;6:458‐73. - PubMed
Unverferth 1983a {published data only}
-
- Unverferth DV, Fertel RH, Balcerzak SP, Magorien RD, O'Dorisio MS. N‐Acetylcysteine prevents the doxorubicin‐induced decrease of cyclic GMP. Seminars in Oncology 1983;10 Suppl 1(1):49‐52. - PubMed
Unverferth 1983b {published data only}
-
- Unverferth DV, Jagadeesh JM, Unverferth BJ, Magorien RD, Leier CV, Balcerzak SP. Attempt to prevent doxorubicin‐induced acute human myocardial morphologic damage with acetylcysteine. Journal of the National Cancer Institute 1983;71:917‐20. - PubMed
Vici 1998 {published data only}
-
- Vici P, Ferraironi A, Lauro L, Carpano S, Conti F, Belli F, et al. Dexrazoxane cardioprotection in advanced breast cancer patients undergoing high‐dose epirubicin treatment. La Clinica Terapeutica 1998;149:15‐20. - PubMed
Wagdi 1996 {published data only}
-
- Wagdi P, Fluri M, Aeschbacher B, Fikrle A, Meier B. Cardioprotection in patients undergoing chemo‐and/or radiotherapy for neoplastic disease: a pilot study. Japanese Heart Journal 1996;37:353‐9. - PubMed
Weisberg 1992 {published data only}
-
- Weisberg SR, Rosenfeld CS, York RM, Jones SE, Spicer DV, Khojasteh A, et al. Dexrazoxane (ADR‐529, ICRF‐187, Zinecard) protects against doxorubicin induced chronic cardiotoxicity. Proceedings of ASCO 1992;11:91.
Weitzman 1980 {published data only}
-
- Weitzman SA, Lorell B, Carey RW, Kaufman S, Stossel TP. Prospective study of tocopherol prophylaxis for anthracycline cardiac toxicity. Current Therapeutic Research 1980;28:682‐6.
Whittaker 1984 {published data only}
Whittaker 1987 {published data only}
-
- Whittaker JA, Al‐Ismail SAD, Nicholson DM, Saour JN. Doxorubicin cardiotoxicity: attempts at prevention with digoxin and alpha‐tocopherol (vitamin E). Clinical Trials Journal 1987;24 Suppl 1:45‐55.
References to studies awaiting assessment
Cadeddu 2010 {published data only (unpublished sought but not used)}
-
- Cadeddu C, Piras A, Mantovani G, Deidda M, Dessì M, Madeddu C, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin‐induced inflammation, oxidative stress, and early ventricular impairment. American Heart Journal 2010;160:487.e1‐7. - PubMed
Cardenas 2003 {published data only}
-
- Cardenas R, Mercado CG, Rivela LR, Calderon EC. Effectiveness and safety of dexrazoxane as a cardioprotector in Mexican children with cancer. Medical and Pediatric Oncology 2003;41:PL001.
De Berranger 2006 {published data only}
-
- Berranger E. Results of randomized study on dexrazoxane administration in children with de novo acute leukemia. ASCO Annual Meeting (abstract number 9037). 2006.
Feldmann 1992 {published data only}
-
- Feldmann JE, Jones SE, Weisberg SR, Gandars DR, Lyman GH, York RM, et al. Advanced small cell lung cancer treated with CAV (cyclophosphamide + adriamycin + vincristine) chemotherapy and the cardioprotective agent dexrazoxane (ADR‐529, ICRF‐187, Zinecard). ASCO Annual Meeting (abstract number 993). 1992.
Gu 2010 {published data only (unpublished sought but not used)}
-
- Gu H‐S, Li J‐Y, Zhang W‐H, Zhang Y, Jia S, Fu Y. Clinical observation of hydroprednisone and glutathione in chemotherapy of breast cancer. Chinese Journal of Cancer Prevention and Treatment 2010;17:131‐3.
Jackowska 2003 {published data only}
-
- Jackowska T, Rokicka‐Milewska R, Matysiak M, Balwierz W, Kowalczyk J, Wachowiak J, et al. Assessment of dexrazoxane tolerance in leukemic children receiving anthracyclines. Medical and Pediatric Oncology 2003;41:PD003.
Saad El‐Din 2003 {published data only}
-
- Saad El‐Din I, Saban K, Abdel Rahman K, Gabaly M. A randomized clinical trial to evaluate cardioprotective effect of ICRF‐187 in children with acute lymphoblastic leukemia. Medical and Pediatric Oncology 2003;41:O109.
Ten Bokkel‐Huinink 1992 {published data only}
-
- Bokkel‐Huinink WW, Schreuder JE, Dubbelman R, Bierhorst F, Tinteren H, Dalesio O, et al. ICRF 187 protects against doxorubicin induced cardiomyopathy. Annals of Oncology (abstract number 221) 1992:114.
References to ongoing studies
NCT00002827/POG9426 {published data only}
-
- Phase III randomised study of response‐dependent therapy with doxorubicin/bleomycin/vincristine/etoposide (DBVE) with versus without dexrazoxane followed by low‐dose involved‐field radiotherapy for newly diagnosed stage IA/IIA/IIIA1 childhood Hodgkin's disease. www.controlled‐trials.com.
NCT00016276 {published data only}
-
- Phase III randomised study of doxorubicin and cyclophosphamide with or without dexrazoxane followed by paclitaxel with or without trastuzumab (herceptin) followed by surgery and radiotherapy with or without trastuzumab in women with HER‐2+ stage IIIA or IIIB or regional stage IV breast cancer. www.controlled‐trials.com.
NCT00162955 {published data only}
-
- The multi‐centers trial for patients with Non‐Hodgkin's lymphoma to assess the protective effect of valsartan on chronic cardiotoxicity induced by CHOP. www.controlled‐trials.com.
NCT00247975 {published data only}
-
- Primary prevention of anthracycline‐induced cardiotoxicity with L‐carnitine in patients with breast cancer (PPACC)‐pilot study. www.controlled‐trials.com.
NCT00895414 {published and unpublished data}
-
- Enalapril maleate and doxorubicin hydrochloride in treating women with breast cancer. www.controlled‐trials.com.
NCT01230983/POG9404 {published data only}
-
- Intensive treatment for T‐cell acute lymphoblastic leukemia and advanced stage lymphoblastic Non‐Hodgkin's lymphoma: a Pediatric Oncology Group Phase III study. www.controlled‐trials.com.
Additional references
Barry 2008
-
- Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high‐risk acute lymphoblastic leukemia treated with dexrazoxane. Journal of Clinical Oncology 2008;26:1106‐11. - PubMed
Batist 2001
-
- Batist G, Ramakriskan G, Sekhar Rao C, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome‐encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized multicenter trial of metastatic breast cancer. Journal of Clinical Oncology 2001;19:1444‐54. - PubMed
Bonadonna 1969
-
- Bonadonna G, Morfandini S. Cardiac toxicity of daunorubicin. Lancet 1969;1:837. - PubMed
De Leonardis 1985
-
- Leonardis V, Neri B, Bacalli S, Cinelli P. Reduction of cardiac toxicity of anthracyclines by L‐Carnitine: preliminary overview of clinical data. International Journal of Clinical Pharmacology Research 1985;5:137‐42. - PubMed
Elihu 1998
-
- Elihu N, Anandasbapathy S, Frishman WH. Chelation therapy in cardiovascular disease: ethylenediaminetetraacetic acid, deferoxamine and dexrazoxane. Journal of Clinical Pharmacology 1998;38:101‐5. - PubMed
Garbrecht 1986
-
- Garbrecht M, Müllerleile U. Verapamil in the prevention of adriamycin‐induced cardiomyopathy. Klinische Wochenschrift 1986;64 Suppl VII:132‐4. - PubMed
Guthrie 1977
Higgins 2006
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated September 2006]. John Wiley & Sons, Ltd, In: The Cochrane Library, Issue 4, 2006. Chichester, UK.
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org, 2008.
Hofmann 1993
-
- Hofmann S, Tuchler H, Bernhart M, Stacher A, Lutz D. A questionnaire suitable for general practice for detection of the health status and quality of life of patients with hemato‐oncologic diseases: psychometric properties. The study G "quality of life" of the International Society for Chemo‐and Immunotherapy (I.G.C.I.). Wiener Klinische Wochenschrift 1993;105:277‐83. - PubMed
Kawasaki 1992
Keizer 1990
-
- Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): a critical review of free radical‐dependent mechanisms of cytotoxicity. Pharmacology and Therapeutics 1990;47:219‐31. - PubMed
Kremer 2002a
-
- Kremer LCM, Pal HJH, Offringa M, Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Annals of Oncology 2002;13:819‐29. - PubMed
Kremer 2002b
-
- Kremer LCM, Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline‐induced heart failure in children: a systematic review. Annals of Oncology 2002;13:503‐12. - PubMed
Lefrak 1973
-
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302‐14. - PubMed
Legha 1982a
-
- Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Annals of Internal Medicine 1982;96:133‐9. - PubMed
Legha 1982b
-
- Legha SS, Wang Y, Mackay B, Ewer M, Hortobagyi GN, Benjamin RS, et al. Clinical and pharmacologic investigation of the effects of a‐tocopherol on adriamycin cardiotoxicity. Annals of the New York Academy of Sciences 1982;393:411‐8. - PubMed
Lipshultz 1998
-
- Lipshultz SE, Sallan SE, Giantris AL, Lipsitz SR, Dalton V, Colan SD. 48 hour continuous doxorubicin infusion is not cardioprotective in children assessed 18 months later: the DFCI 91001 ALL protocol. Proceedings of the American Society of Clinical Oncology 1998;17:528a.
Lipshultz 2010
-
- Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin‐treated children with high‐risk acute lymphoblastic leukaemia: long‐term follow‐up of a prospective, randomised, multicentre trial. Lancet Oncology 2010;11:950‐61. - PMC - PubMed
Miller 1981
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207‐14. - PubMed
Muggia 1991
-
- Muggia FM, Green MD. New anthracycline antitumor antibiotics. Critical Reviews in Oncology/Hematology 1991;11:43‐64. - PubMed
Muggia 1997
-
- Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. Journal of Clinical Oncology 1997;15:987‐93. - PubMed
Myers 1998
-
- Myers C. The role of iron in doxorubicin‐induced cardiomyopathy. Seminars in Oncology 1998;25 Suppl 10:10‐4. - PubMed
Odaimi 1987
-
- Odaimi M, Ajani J. High‐dose chemotherapy. American Journal of Clinical Oncology 1987;10:123‐32. - PubMed
Oken 1982
-
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5:649‐55. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Pierga 2001
-
- Pierga JY, Robain M, Jouve M, Asselain B, Dieras V, Beuzeboc P, et al. Response to chemotherapy is a major parameter‐influencing long‐term survival of metastatic breast cancer patients. Annals of Oncology 2001;12:231‐7. - PubMed
Salzer 2010
Shan 1996
-
- Shan K, Lincoff AM, Young JB. Anthracycline‐induced cardiotoxicity. Annals of Internal Medicine 1996;125:47‐58. - PubMed
Silber 2001
-
- Silber JH, Cnaan A, Clark BJ, Paridon SM, Chin AJ, Rychik J, et al. Design and baseline characteristics for the ACE inhibitor after anthracycline (AAA) study of cardiac dysfunction in long‐term pediatric cancer survivors. American Heart Journal 2001;142:577‐85. - PubMed
Singal 1995
-
- Singal PK, Siveski‐Iliskovic N, Hill M, Thomas TP, Li T. Combination therapy with probucol prevents adriamycin‐induced cardiomyopathy. Journal of Molecular and Cellular Cardiology 1995;27:1055‐62. - PubMed
Tuchler 1992
-
- Tuchler H, Hofmann S, Bernhart M, Brugiatelli M, Chrobak L, Franke A, et al. A short multilingual quality of life questionnaire ‐ practicability, reliability and interlingual homogeneity. Quality of Life Research 1992;1:107‐17. - PubMed
Van Acker 2000
-
- Acker FAA, Acker SABE, Kramer K, Haenen GR, Bast A, Vijgh WJ. 7‐monohydroxyethylrutoside protects against chronic doxorubicin‐induced cardiotoxicity when administered only once per week. Clinical Cancer Research 2000;6:1337‐41. - PubMed
Van Dalen 2009
-
- Dalen EC, Pal HJ, Caron HN, Kremer LC. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005008.pub2] - DOI
Van Dalen 2010
Van Dalen 2011
-
- Dalen EC, Berg H, Raphaël MF, Caron HN, Kremer LC. Should anthracyclines and dexrazoxane be used for children with cancer?. Lancet Oncology 2011;12(1):12‐3. - PubMed
Von Hoff 1979
-
- Hoff DD, Layard MW, Basa P, Davis HL Jr, Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin‐induced congestive heart failure. Annals of Internal Medicine 1979;91:710‐7. - PubMed
References to other published versions of this review
Van Dalen 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

