Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment
- PMID: 21678528
- PMCID: PMC3412675
- DOI: 10.1002/stem.674
Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment
Abstract
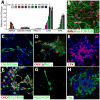
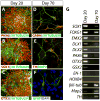
Differentiation methods for human induced pluripotent stem cells (hiPSCs) typically yield progeny from multiple tissue lineages, limiting their use for drug testing and autologous cell transplantation. In particular, early retina and forebrain derivatives often intermingle in pluripotent stem cell cultures, owing to their shared ancestry and tightly coupled development. Here, we demonstrate that three-dimensional populations of retinal progenitor cells (RPCs) can be isolated from early forebrain populations in both human embryonic stem cell and hiPSC cultures, providing a valuable tool for developmental, functional, and translational studies. Using our established protocol, we identified a transient population of optic vesicle (OV)-like structures that arose during a time period appropriate for normal human retinogenesis. These structures were independently cultured and analyzed to confirm their multipotent RPC status and capacity to produce physiologically responsive retinal cell types, including photoreceptors and retinal pigment epithelium (RPE). We then applied this method to hiPSCs derived from a patient with gyrate atrophy, a retinal degenerative disease affecting the RPE. RPE generated from these hiPSCs exhibited a disease-specific functional defect that could be corrected either by pharmacological means or following targeted gene repair. The production of OV-like populations from human pluripotent stem cells should facilitate the study of human retinal development and disease and advance the use of hiPSCs in personalized medicine.
Copyright © 2011 AlphaMed Press.
Conflict of interest statement
The authors declare competing financial interests: J.A.T. is a founder, stockowner, consultant and board member of Cellular Dynamics International (CDI). He also serves as scientific advisor to and has financial interests in Tactics II Stem Cell Ventures. CDI currently has no products related to the retina. The other authors have no financial interests to disclose.
Figures





References
-
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. - PubMed
-
- Pera MF, Trounson AO. Human embryonic stem cells: prospects for development. Development. 2004;131:5515–5525. - PubMed
-
- Gamm DM, Meyer JS. Directed differentiation of human induced pluripotent stem cells: a retina perspective. Regen Med. 2010;5:315–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

