Effects of electrode surface modification with chlorotoxin on patterning single glioma cells
- PMID: 21678586
- PMCID: PMC3205118
- DOI: 10.1039/c0cp02908d
Effects of electrode surface modification with chlorotoxin on patterning single glioma cells
Abstract
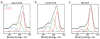
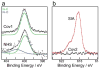
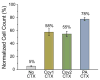
A microchip patterned with arrays of single cancer cells can be an effective platform for the study of tumor biology, medical diagnostics, and drug screening. However, patterning and retaining viable single cancer cells on defined sites of the microarray can be challenging. In this study we used a tumor cell-specific peptide, chlorotoxin (CTX), to mediate glioma cell adhesion on arrays of gold microelectrodes and investigated the effects of three surface modification schemes for conjugation of CTX to the microelectrodes on single cell patterning, which include physical adsorption, covalent bonding mediated by N-hydroxysuccinimide (NHS), and covalent bonding via crosslinking succinimidyl iodoacetate and Traut's (SIA-Traut) reagents. The CTX immobilization to microelectrodes was confirmed by high-resolution X-ray photoelectron spectroscopy. Physically adsorbed CTX showed better support for cell adhesion and is more effective in confining adhered cells on the electrodes than covalently-bound CTX. Furthermore, cell adhesion and spreading on microelectrodes were quantified in real-time by impedance measurements, which revealed an impedance signal from physically adsorbed CTX electrodes four times greater than the signal from covalently-bound CTX electrodes.
© The Owner Societies 2011
Figures
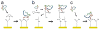


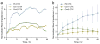
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

