Catalytic asymmetric intermolecular Stetter reaction of enals with nitroalkenes: enhancement of catalytic efficiency through bifunctional additives
- PMID: 21678918
- PMCID: PMC3147302
- DOI: 10.1021/ja203810b
Catalytic asymmetric intermolecular Stetter reaction of enals with nitroalkenes: enhancement of catalytic efficiency through bifunctional additives
Abstract
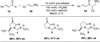
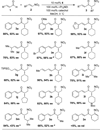
An asymmetric intermolecular Stetter reaction of enals with nitroalkenes catalyzed by chiral N-heterocyclic carbenes has been developed. The reaction rate and efficiency are profoundly impacted by the presence of catechol. The reaction proceeds with high selectivities and affords good yields of the Stetter product. Internal redox products were not observed despite of the protic conditions. The impact of catechol has been found to be general, facilitating far lower catalyst loadings than were previously achievable.
Figures
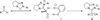





References
-
-
For reviews see: Marion N, Díez-González S, Nolan SP. Angew. Chem. Int. Ed. Engl. 2007;46:2988–3000. Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606–5655. Moore JL, Rovis T. Top. Curr. Chem. 2009;290:77–144.
-
-
-
Stetter H, Schrecke M. Angew. Chem., Int. Ed. Engl. 1973;12:81. Stetter H. Angew. Chem., Int. Ed. Engl. 1976;15:639–647. Stetter H, Kuhlmann H. Org. React. 1991;40:407–496. Christmann M. Angew. Chem., Int. Ed. 2005;44:2632–2634. Rovis T. Chem. Lett. 2008;37:2–7.. For a review of the asymmetric intramolecular Stetter reaction see: Read de Alaniz J, Rovis T. Synlett. 2009:1189–1207.. For a general review on Lewis base catalysis, see: Denmark SE, Beutner GL. Angew. Chem. Int. Ed. 2008;47:1560–1638.
-
-
-
DiRocco DA, Oberg KM, Dalton DM, Rovis T. J. Am. Chem. Soc. 2009;131:10872–10874.. For review of fluorine’s effect on molecular conformation, see: Hunter L. Beilstein J. Org. Chem. 2010 doi:10.3762/bjoc.6.38.
-
-
-
For other contributions to the asymmetric intermolecular Stetter reaction, see: Liu Q, Perreault S, Rovis T. J. Am. Chem. Soc. 2008;130:14066–14067. Liu Q, Rovis T. Org. Lett. 2009;11:2856–2859. Enders D, Han J, Henseler A. Chem. Commun. 2008:3989–3991. Enders D, Han J. Synthesis. 2008:3864–3868. Jousseaume T, Wurz NE, Glorius F. Angew. Chem. Int. Ed. 2011;50:1410–1414.. (f) For an enzyme-catalyzed asymmetric Stetter, see: Dresen C, Richter M, Pohl M, Lüdeke S, Müller M. Angew. Chem. Int. Ed. 2010;49:6600–6603.
-
-
-
Evidence suggests that the role of the heteroatom is not simply that of a proximal Lewis base given that both pyridazine carboxaldehyde and furfural participate with equal facility in spite of their very low basicity. See reference .
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

