A randomized controlled trial of emergency treatment of bleeding esophageal varices in cirrhosis for hepatocellular carcinoma
- PMID: 21679921
- PMCID: PMC6369695
- DOI: 10.1016/j.amjsurg.2011.02.007
A randomized controlled trial of emergency treatment of bleeding esophageal varices in cirrhosis for hepatocellular carcinoma
Abstract
Background: Ninety percent of patients with hepatocellular carcinoma (HCC) have cirrhosis. Bleeding esophageal varices (BEV) is a frequent complication of cirrhosis. Detection of HCC in cirrhotic patients with BEV has not been studied.
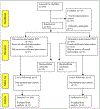
Methods: Two hundred eleven unselected patients with cirrhosis and BEV were randomized to endoscopic sclerotherapy (n = 106) or emergency portacaval shunt (n = 105). Diagnostic workup and treatment were initiated within 8 hours. Ninety-six percent had >10 years of follow-up. HCC screening involved serum α-fetoprotein (AFP) every 3 months, ultrasonography every 6 months, and selective computed tomography (CT).
Results: HCC occurred in 15 patients, all incurable, a mean of 2.94 years after entry. They died a mean 1.33 years after discovery. Serial AFP and ultrasound examinations were unrevealing over a mean of 2.3 years. The mean model of end-stage liver disease score was 12.7 at entry and 17.4 at HCC diagnosis.
Conclusions: Long-term screening by AFP and ultrasound plus selective CT failed to detect HCC at a curable stage. The detection of HCC in cirrhotic patients with BEV remains a serious, unsolved problem. The use of CT for routine screening warrants consideration despite increased costs.
Trial registration: ClinicalTrials.gov NCT00690027.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907–17. - PubMed
-
- El-Serag HB. Should we screen for hepatocellular carcinoma in patients with cirrhosis? If so, in whom and how? AGA Perspect 2010; 6:20–1.
-
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–50. - PubMed
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–76. - PubMed
-
- Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127:S5–16. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical