FosB is essential for the enhancement of stress tolerance and antagonizes locomotor sensitization by ΔFosB
- PMID: 21679928
- PMCID: PMC3264950
- DOI: 10.1016/j.biopsych.2011.04.021
FosB is essential for the enhancement of stress tolerance and antagonizes locomotor sensitization by ΔFosB
Erratum in
- Biol Psychiatry. 2012 Sep 1;72(5):429
Abstract
Background: Molecular mechanisms underlying stress tolerance and vulnerability are incompletely understood. The fosB gene is an attractive candidate for regulating stress responses, because ΔFosB, an alternative splice product of the fosB gene, accumulates after repeated stress or antidepressant treatments. On the other hand, FosB, the other alternative splice product of the fosB gene, expresses more transiently than ΔFosB but exerts higher transcriptional activity. However, the functional differences of these two fosB products remain unclear.
Methods: We established various mouse lines carrying three different types of fosB allele, wild-type (fosB(+)), fosB-null (fosB(G)), and fosB(d) allele, which encodes ΔFosB but not FosB, and analyzed them in stress-related behavioral tests.
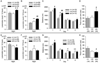
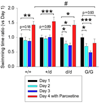
Results: Because fosB(+/d) mice show enhanced ΔFosB levels in the presence of FosB and fosB(d/d) mice show more enhanced ΔFosB levels in the absence of FosB, the function of FosB can be inferred from differences observed between these lines. The fosB(+/d) and fosB(d/d) mice showed increased locomotor activity and elevated Akt phosphorylation, whereas only fosB(+/d) mice showed antidepressive-like behaviors and increased E-cadherin expression in striatum compared with wild-type mice. In contrast, fosB-null mice showed increased depression-like behavior and lower E-cadherin expression.
Conclusions: These findings indicate that FosB is essential for stress tolerance mediated by ΔFosB. These data suggest that fosB gene products have a potential to regulate mood disorder-related behaviors.
Copyright © 2011 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures




References
-
- Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. - PubMed
-
- Kurushima H, Ohno M, Miura T, Nakamura TY, Horie H, Kadoya T, et al. Selective induction of ΔFosB in the brain after transient forebrain ischemia accompanied by an increased expression of galectin-1, and the implication of ΔFosB and galectin-1 in neuroprotection and neurogenesis. Cell Death Differ. 2005;12:1078–1096. - PubMed
-
- Miura T, Ohnishi Y, Kurushima H, Horie H, Kadoya T, Nakabeppu Y. Regulation of the neuronal fate by ΔFosB and its downstream target, galectin-1. Current Drug Targets. 2005;6:437–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

