Centrosomes and cilia in human disease
- PMID: 21680046
- PMCID: PMC3144269
- DOI: 10.1016/j.tig.2011.05.004
Centrosomes and cilia in human disease
Abstract
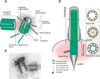
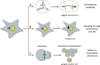
Centrioles are microtubule-derived structures that are essential for the formation of centrosomes, cilia and flagella. The centrosome is the major microtubule organiser in animal cells, participating in a variety of processes, from cell polarisation to cell division, whereas cilia and flagella contribute to several mechanisms in eukaryotic cells, from motility to sensing. Although it was suggested more than a century ago that these microtubule-derived structures are involved in human disease, the molecular bases of this association have only recently been discovered. Surprisingly, there is very little overlap between the genes affected in the different diseases, suggesting that there are tissue-specific requirements for these microtubule-derived structures. Knowledge of these requirements and disease mechanisms has opened new avenues for therapeutical strategies. Here, we give an overview of recent developments in this field, focusing on cancer, diseases of brain development and ciliopathies.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–463. - PubMed
-
- Conduit PT, Raff JW. Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in drosophila neuroblasts. Curr Biol. 2010;20:2187–2192. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

