Preservation of acute pain and efferent functions following intrathecal resiniferatoxin-induced analgesia in rats
- PMID: 21680254
- PMCID: PMC3645374
- DOI: 10.1016/j.jpain.2011.03.005
Preservation of acute pain and efferent functions following intrathecal resiniferatoxin-induced analgesia in rats
Abstract
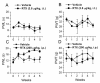
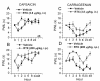
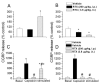
Resiniferatoxin (RTX) is a potent agonist of TRPV1, which possesses unique properties that can be utilized to treat certain modalities of pain. In the present study, systemic intraperitoneal (i.p.) administration of RTX resulted in a significant decrease in acute thermal pain sensitivity, whereas localized intrathecal (i.t.) administration had no effect on acute thermal pain sensitivity. Both i.p. and i.t. administration of RTX prevented TRPV1-induced nocifensive behavior and inflammatory thermal hypersensitivity. There were no alterations in mechanical sensitivity either by i.p. or i.t. administration of RTX. In spinal dorsal horn (L4-L6), TRPV1 and substance P immunoreactivity were abolished following i.p. and i.t. administration of RTX. In dorsal root ganglia (DRG), TRPV1 immunoreactivity was diminished following i.p. administration, but was unaffected following i.t. administration of RTX. Following i.p. administration, basal and evoked calcitonin gene-related peptide release were reduced both in the spinal cord and peripheral tissues. However, following i.t. administration, basal and evoked calcitonin gene-related peptide release were reduced in spinal cord (L4-L6), but were unaffected in peripheral tissues. Both i.p. and i.t. RTX administration lowered the body temperature acutely, but this effect reversed with time. Targeting TRPV1-expressing nerve terminals at the spinal cord can selectively abolish inflammatory thermal hypersensitivity without affecting acute thermal sensitivity and can preserve the efferent functions of DRG neurons at the peripheral nerve terminals. I.t. administration of RTX can be considered as a strategy for treating certain chronic and debilitating pain conditions.
Perspective: Localized administration of RTX in spinal cord could be a useful strategy to treat chronic debilitating pain arising from certain conditions such as cancer and at the same time could maintain normal physiological peripheral efferent functions mediated by TRPV1.
Copyright © 2011 American Pain Society. Published by Elsevier Inc. All rights reserved.
Figures



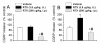
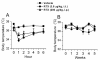
References
-
- Ayoub SS, Hunter JC, Simmons DL. Answering the burning question of how transient receptor potential vanilloid-1 channel antagonists cause unwanted hyperthermia. Pharmacol Rev. 2009;61:225–7. - PubMed
-
- Bishnoi M, Premkumar LS. Possible Consequences of Blocking Transient Receptor Potential Vanilloid 1. Curr Pharm Biotechnol. 2010 [Epub ahead of print] - PubMed
-
- Bretag A. Synthetic interstitial fluid for isolated mammalian tissue. Life Sci. 1969;8:319–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

