Nuclear envelope breakdown can substitute for primary envelopment-mediated nuclear egress of herpesviruses
- PMID: 21680518
- PMCID: PMC3147978
- DOI: 10.1128/JVI.00741-11
Nuclear envelope breakdown can substitute for primary envelopment-mediated nuclear egress of herpesviruses
Abstract
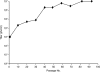
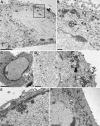
Herpesvirus nucleocapsids assemble in the nucleus but mature to infectious virions in the cytoplasm. To gain access to this cellular compartment, nucleocapsids are translocated to the cytoplasm by primary envelopment at the inner nuclear membrane and subsequent fusion of the primary envelope with the outer nuclear membrane. The conserved viral pUL34 and pUL31 proteins play a crucial role in this process. In their absence, viral replication is strongly impaired but not totally abolished. We used the residual infectivity of a pUL34-deleted mutant of the alphaherpesvirus pseudorabies virus (PrV) for reversion analysis. To this end, PrV-ΔUL34 was serially passaged in rabbit kidney cells until final titers of the mutant virus PrV-ΔUL34Pass were comparable to those of wild-type PrV. PrV-ΔUL34Pass produced infectious progeny independently of the pUL34/pUL31 nuclear egress complex and the pUS3 protein kinase. Ultrastructural analyses demonstrated that this effect was due to virus-induced disintegration of the nuclear envelope, thereby releasing immature and mature capsids into the cytosol for secondary envelopment. Our data indicate that nuclear egress primarily serves to transfer capsids through the intact nuclear envelope. Immature and mature intranuclear capsids are competent for further virion maturation once they reach the cytoplasm. However, nuclear egress exhibits a strong bias for nucleocapsids, thereby also functioning as a quality control checkpoint which is abolished by herpesvirus-induced nuclear envelope breakdown.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

