Dynamic profiling of mRNA turnover reveals gene-specific and system-wide regulation of mRNA decay
- PMID: 21680716
- PMCID: PMC3145553
- DOI: 10.1091/mbc.E11-01-0028
Dynamic profiling of mRNA turnover reveals gene-specific and system-wide regulation of mRNA decay
Abstract
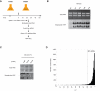
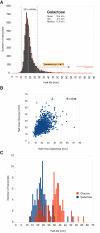
RNA levels are determined by the rates of both transcription and decay, and a mechanistic understanding of the complex networks regulating gene expression requires methods that allow dynamic measurements of transcription and decay in living cells with minimal perturbation. Here, we describe a metabolic pulse-chase labeling protocol using 4-thiouracil combined with large-scale RNA sequencing to determine decay rates of all mRNAs in Saccharomyces cerevisiae. Profiling in various growth and stress conditions reveals that mRNA turnover is highly regulated both for specific groups of transcripts and at the system-wide level. For example, acute glucose starvation induces global mRNA stabilization but increases the degradation of all 132 detected ribosomal protein mRNAs. This effect is transient and can be mimicked by inhibiting the target-of-rapamycin kinase. Half-lives of mRNAs critical for galactose (GAL) metabolism are also highly sensitive to changes in carbon source. The fast reduction of GAL transcripts in glucose requires their dramatically enhanced turnover, highlighting the importance of mRNA decay in the control of gene expression. The approach described here provides a general platform for the global analysis of mRNA turnover and transcription and can be applied to dissect gene expression programs in a wide range of organisms and conditions.
Figures
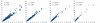
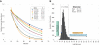
References
-
- Boeke JD, Garfinkel DJ, Styles CA, Fink GR. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. - PubMed
-
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

