The role of trimerization in the osmoregulated betaine transporter BetP
- PMID: 21681199
- PMCID: PMC3147255
- DOI: 10.1038/embor.2011.102
The role of trimerization in the osmoregulated betaine transporter BetP
Abstract
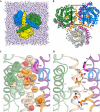
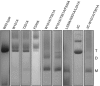
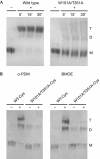
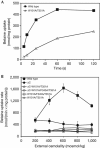
The osmoregulated betaine transporter BetP is a stable trimer. Structural studies have shown that individual protomers can adopt distinct transport conformations, implying a functional role for the trimeric state in transport, although the role of trimerization in regulation is not yet understood. We designed putative monomeric mutants by molecular-dynamics simulations and in silico alanine-scanning mutagenesis. Several mutants including BetP-W101A/T351A were monomeric in detergent as well as in the membrane, as shown by blue native gel electrophoresis, crosslinking and electron microscopy. This monomeric form retains the ability to accumulate betaine, but is no longer regulated by hyperosmotic shock.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Eicher T, Brandstätter L, Pos KM (2009) Structural and functional aspects of the multidrug efflux pump AcrB. Biol Chem 390: 693–699 - PubMed
-
- Faraldo-Gómez JD, Smith GR, Sansom MS (2002) Setting up and optimization of membrane protein simulations. Eur Biophys J 31: 217–227 - PubMed
-
- Forrest L, Krämer R, Ziegler C (2011) The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807: 167–188 - PubMed
-
- Herz K, Rimon A, Jeschke G, Padan E (2009) β-Sheet-dependent dimerization is essential for the stability of NhaA Na+/H+ antiporter. J Biol Chem 284: 6337–6347 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

