N-carbamylglutamate enhancement of ureagenesis leads to discovery of a novel deleterious mutation in a newly defined enhancer of the NAGS gene and to effective therapy
- PMID: 21681857
- PMCID: PMC3976964
- DOI: 10.1002/humu.21553
N-carbamylglutamate enhancement of ureagenesis leads to discovery of a novel deleterious mutation in a newly defined enhancer of the NAGS gene and to effective therapy
Abstract
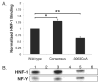
N-acetylglutamate synthase (NAGS) catalyzes the conversion of glutamate and acetyl-CoA to NAG, the essential allosteric activator of carbamyl phosphate synthetase I, the first urea cycle enzyme in mammals. A 17-year-old female with recurrent hyperammonemia attacks, the cause of which remained undiagnosed for 8 years in spite of multiple molecular and biochemical investigations, showed markedly enhanced ureagenesis (measured by isotope incorporation) in response to N-carbamylglutamate (NCG). This led to sequencing of the regulatory regions of the NAGS gene and identification of a deleterious single-base substitution in the upstream enhancer. The homozygous mutation (c.-3064C>A), affecting a highly conserved nucleotide within the hepatic nuclear factor 1 (HNF-1) binding site, was not found in single nucleotide polymorphism databases and in a screen of 1,086 alleles from a diverse population. Functional assays demonstrated that this mutation decreases transcription and binding of HNF-1 to the NAGS gene, while a consensus HNF-1 binding sequence enhances binding to HNF-1 and increases transcription. Oral daily NCG therapy restored ureagenesis in this patient, normalizing her biochemical markers, and allowing discontinuation of alternate pathway therapy and normalization of her diet with no recurrence of hyperammonemia. Inc.
© 2011 Wiley-Liss, Inc.
Figures





Similar articles
-
N-Acetylglutamate Synthase Deficiency Due to a Recurrent Sequence Variant in the N-acetylglutamate Synthase Enhancer Region.Sci Rep. 2018 Oct 18;8(1):15436. doi: 10.1038/s41598-018-33457-0. Sci Rep. 2018. PMID: 30337552 Free PMC article.
-
Gene delivery corrects N-acetylglutamate synthase deficiency and enables insights in the physiological impact of L-arginine activation of N-acetylglutamate synthase.Sci Rep. 2021 Feb 11;11(1):3580. doi: 10.1038/s41598-021-82994-8. Sci Rep. 2021. PMID: 33574402 Free PMC article.
-
Noncoding sequence variants define a novel regulatory element in the first intron of the N-acetylglutamate synthase gene.Hum Mutat. 2021 Dec;42(12):1624-1636. doi: 10.1002/humu.24281. Epub 2021 Sep 24. Hum Mutat. 2021. PMID: 34510628 Free PMC article.
-
N-acetylglutamate synthase: structure, function and defects.Mol Genet Metab. 2010;100 Suppl 1(Suppl 1):S13-9. doi: 10.1016/j.ymgme.2010.02.018. Epub 2010 Feb 26. Mol Genet Metab. 2010. PMID: 20303810 Free PMC article. Review.
-
Presentation and management of N-acetylglutamate synthase deficiency: a review of the literature.Orphanet J Rare Dis. 2020 Oct 9;15(1):279. doi: 10.1186/s13023-020-01560-z. Orphanet J Rare Dis. 2020. PMID: 33036647 Free PMC article.
Cited by
-
The efficacy of Carbamylglutamate impacts the nutritional management of patients with N-Acetylglutamate synthase deficiency.Orphanet J Rare Dis. 2024 Apr 18;19(1):168. doi: 10.1186/s13023-024-03167-0. Orphanet J Rare Dis. 2024. PMID: 38637895 Free PMC article.
-
Augmenting ureagenesis in patients with partial carbamyl phosphate synthetase 1 deficiency with N-carbamyl-L-glutamate.J Pediatr. 2014 Aug;165(2):401-403.e3. doi: 10.1016/j.jpeds.2014.04.012. Epub 2014 May 29. J Pediatr. 2014. PMID: 24880889 Free PMC article. Clinical Trial.
-
Precision medicine in rare disease: Mechanisms of disparate effects of N-carbamyl-l-glutamate on mutant CPS1 enzymes.Mol Genet Metab. 2017 Mar;120(3):198-206. doi: 10.1016/j.ymgme.2016.12.002. Epub 2016 Dec 8. Mol Genet Metab. 2017. PMID: 28007335 Free PMC article.
-
Amino Acid Metabolism in Liver Mitochondria: From Homeostasis to Disease.Metabolites. 2025 Jul 2;15(7):446. doi: 10.3390/metabo15070446. Metabolites. 2025. PMID: 40710547 Free PMC article. Review.
-
NAGS, CPS1, and SLC25A13 (Citrin) at the Crossroads of Arginine and Pyrimidines Metabolism in Tumor Cells.Int J Mol Sci. 2023 Apr 4;24(7):6754. doi: 10.3390/ijms24076754. Int J Mol Sci. 2023. PMID: 37047726 Free PMC article.
References
-
- Bachmann C, Brandis M, Weissenbarth-Riedel E, Burghard R, Colombo JP. N-acetylglutamate synthetase deficiency, a second patient. J Inherit Metab Dis. 1988;11(2):191–3. - PubMed
-
- Bachmann C, Krahenbuhl S, Colombo JP, Schubiger G, Jaggi KH, Tonz O. N-acetylglutamate synthetase deficiency: a disorder of ammonia detoxication. N Engl J Med. 1981;304(9):543. - PubMed
-
- Batshaw ML. Hyperammonemia. Curr Probl Pediatr. 1984;14(11):1–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

