Programming microbial population dynamics by engineered cell-cell communication
- PMID: 21681967
- PMCID: PMC3697107
- DOI: 10.1002/biot.201100132
Programming microbial population dynamics by engineered cell-cell communication
Abstract
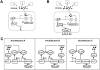
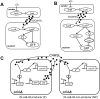
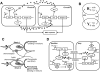
A major aim of synthetic biology is to program novel cellular behavior using engineered gene circuits. Early endeavors focused on building simple circuits that fulfill simple functions, such as logic gates, bistable toggle switches, and oscillators. These gene circuits have primarily focused on single-cell behaviors since they operate intracellularly. Thus, they are often susceptible to cell-cell variations due to stochastic gene expression. Cell-cell communication offers an efficient strategy to coordinate cellular behavior at the population level. To this end, we review recent advances in engineering cell-cell communication to achieve reliable population dynamics, spanning from communication within single species to multispecies, from one-way sender-receiver communication to two-way communication in synthetic microbial ecosystems. These engineered systems serve as well-defined model systems to better understand design principles of their naturally occurring counterparts and to facilitate novel biotechnology applications.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures




Similar articles
-
Synthetic genetic circuits for programmable biological functionalities.Biotechnol Adv. 2019 Nov 1;37(6):107393. doi: 10.1016/j.biotechadv.2019.04.015. Epub 2019 Apr 30. Biotechnol Adv. 2019. PMID: 31051208 Review.
-
Engineered cell-cell communication and its applications.Adv Biochem Eng Biotechnol. 2014;146:97-121. doi: 10.1007/10_2013_249. Adv Biochem Eng Biotechnol. 2014. PMID: 24002441 Review.
-
Engineering microbial consortia with rationally designed cellular interactions.Curr Opin Biotechnol. 2022 Aug;76:102730. doi: 10.1016/j.copbio.2022.102730. Epub 2022 May 21. Curr Opin Biotechnol. 2022. PMID: 35609504 Free PMC article. Review.
-
Quorum Sensing Communication Modules for Microbial Consortia.ACS Synth Biol. 2016 Sep 16;5(9):969-77. doi: 10.1021/acssynbio.5b00286. Epub 2016 May 19. ACS Synth Biol. 2016. PMID: 27172092 Free PMC article.
-
Quorum Sensing System Used as a Tool in Metabolic Engineering.Biotechnol J. 2020 Jun;15(6):e1900360. doi: 10.1002/biot.201900360. Epub 2020 Mar 12. Biotechnol J. 2020. PMID: 32034863 Review.
Cited by
-
Directed evolution: an evolving and enabling synthetic biology tool.Curr Opin Chem Biol. 2012 Aug;16(3-4):285-91. doi: 10.1016/j.cbpa.2012.05.186. Epub 2012 Jun 4. Curr Opin Chem Biol. 2012. PMID: 22673064 Free PMC article. Review.
-
Sender-receiver systems and applying information theory for quantitative synthetic biology.Curr Opin Biotechnol. 2015 Feb;31:101-7. doi: 10.1016/j.copbio.2014.08.005. Epub 2014 Oct 1. Curr Opin Biotechnol. 2015. PMID: 25282688 Free PMC article. Review.
-
Enhancing E. coli tolerance towards oxidative stress via engineering its global regulator cAMP receptor protein (CRP).PLoS One. 2012;7(12):e51179. doi: 10.1371/journal.pone.0051179. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23251448 Free PMC article.
-
Key amino acid residues govern the substrate selectivity of the transporter Xltr1p from Trichoderma reesei for glucose, mannose, and galactose.Eng Microbiol. 2024 May 22;4(4):100151. doi: 10.1016/j.engmic.2024.100151. eCollection 2024 Dec. Eng Microbiol. 2024. PMID: 39628594 Free PMC article.
-
Coupling Cell Communication and Optogenetics: Implementation of a Light-Inducible Intercellular System in Yeast.ACS Synth Biol. 2023 Jan 20;12(1):71-82. doi: 10.1021/acssynbio.2c00338. Epub 2022 Dec 19. ACS Synth Biol. 2023. PMID: 36534043 Free PMC article.
References
-
- Drubin DA, Way JC, Silver PA. Designing biological systems. Genes Dev. 2007;21:242–254. - PubMed
-
- Chang MC, Keasling JD. Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol. 2006;2:674–681. - PubMed
-
- Fortman JL, Chhabra S, Mukhopadhyay A, Chou H, et al. Biofuel alternatives to ethanol: pumping the microbial well. Trends Biotechnol. 2008;26:375–381. - PubMed
-
- Pieper DH, Reineke W. Engineering bacteria for bioremediation. Curr Opin Biotechnol. 2000;11:262–270. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

