Dramatic destabilization of transmembrane helix interactions by features of natural membrane environments
- PMID: 21682279
- PMCID: PMC3140635
- DOI: 10.1021/ja204524c
Dramatic destabilization of transmembrane helix interactions by features of natural membrane environments
Abstract
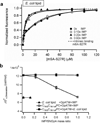
Membrane proteins have evolved to fold and function in a lipid bilayer, so it is generally assumed that their stability should be optimized in a natural membrane environment. Yet optimal stability is not always in accord with optimization of function, so evolutionary pressure, occurring in a complex membrane environment, may favor marginal stability. Here, we find that the transmembrane helix dimer, glycophorin A (GpATM), is actually much less stable in the heterogeneous environment of a natural membrane than it is in model membranes and even common detergents. The primary destabilizing factors are electrostatic interactions between charged lipids and charged GpATM side chains, and nonspecific competition from other membrane proteins. These effects overwhelm stabilizing contributions from lateral packing pressure and excluded volume. Our work illustrates how evolution can employ membrane composition to modulate protein stability.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

