Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement
- PMID: 21683323
- PMCID: PMC3135815
- DOI: 10.1016/j.ajhg.2011.05.015
Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement
Abstract
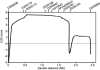
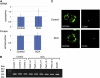
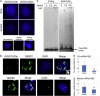
Autosomal-dominant spinocerebellar ataxias (SCAs) are a heterogeneous group of neurodegenerative disorders. In this study, we performed genetic analysis of a unique form of SCA (SCA36) that is accompanied by motor neuron involvement. Genome-wide linkage analysis and subsequent fine mapping for three unrelated Japanese families in a cohort of SCA cases, in whom molecular diagnosis had never been performed, mapped the disease locus to the region of a 1.8 Mb stretch (LOD score of 4.60) on 20p13 (D20S906-D20S193) harboring 37 genes with definitive open reading frames. We sequenced 33 of these and observed a large expansion of an intronic GGCCTG hexanucleotide repeat in NOP56 and an unregistered missense variant (Phe265Leu) in C20orf194, but we found no mutations in PDYN and TGM6. The expansion showed complete segregation with the SCA phenotype in family studies, whereas Phe265Leu in C20orf194 did not. Screening of the expansions in the SCA cohort cases revealed four additional occurrences, but none were revealed in the cohort of 27 Alzheimer disease cases, 154 amyotrophic lateral sclerosis cases, or 300 controls. In total, nine unrelated cases were found in 251 cohort SCA patients (3.6%). A founder haplotype was confirmed in these cases. RNA foci formation was detected in lymphoblastoid cells from affected subjects by fluorescence in situ hybridization. Double staining and gel-shift assay showed that (GGCCUG)n binds the RNA-binding protein SRSF2 but that (CUG)(6) does not. In addition, transcription of MIR1292, a neighboring miRNA, was significantly decreased in lymphoblastoid cells of SCA patients. Our finding suggests that SCA36 is caused by hexanucleotide repeat expansions through RNA gain of function.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Spinocerebellar ataxia type 36 exists in diverse populations and can be caused by a short hexanucleotide GGCCTG repeat expansion.J Neurol Neurosurg Psychiatry. 2015 Sep;86(9):986-95. doi: 10.1136/jnnp-2014-309153. Epub 2014 Dec 4. J Neurol Neurosurg Psychiatry. 2015. PMID: 25476002
-
Clinical features of SCA36: a novel spinocerebellar ataxia with motor neuron involvement (Asidan).Neurology. 2012 Jul 24;79(4):333-41. doi: 10.1212/WNL.0b013e318260436f. Epub 2012 Jun 27. Neurology. 2012. PMID: 22744658
-
Genetic and clinical analysis of spinocerebellar ataxia type 36 in Mainland China.Clin Genet. 2016 Aug;90(2):141-8. doi: 10.1111/cge.12706. Epub 2016 Jan 20. Clin Genet. 2016. PMID: 26661328
-
[Spinocerebellar ataxia type 36 (nicknamed Asidan)].Brain Nerve. 2012 Aug;64(8):937-41. Brain Nerve. 2012. PMID: 22868885 Review. Japanese.
-
Long-read sequencing identified intronic (GGCCTG)n expansion in NOP56 in one SCA36 family and literature review.Clin Neurol Neurosurg. 2022 Dec;223:107503. doi: 10.1016/j.clineuro.2022.107503. Epub 2022 Oct 30. Clin Neurol Neurosurg. 2022. PMID: 36368168 Review.
Cited by
-
RNA toxicity in non-coding repeat expansion disorders.EMBO J. 2020 Jan 2;39(1):e101112. doi: 10.15252/embj.2018101112. Epub 2019 Nov 13. EMBO J. 2020. PMID: 31721251 Free PMC article. Review.
-
RNA toxicity and foci formation in microsatellite expansion diseases.Curr Opin Genet Dev. 2017 Jun;44:17-29. doi: 10.1016/j.gde.2017.01.005. Epub 2017 Feb 14. Curr Opin Genet Dev. 2017. PMID: 28208060 Free PMC article. Review.
-
Using the shared genetics of dystonia and ataxia to unravel their pathogenesis.Neurosci Biobehav Rev. 2017 Apr;75:22-39. doi: 10.1016/j.neubiorev.2017.01.033. Epub 2017 Jan 28. Neurosci Biobehav Rev. 2017. PMID: 28143763 Free PMC article. Review.
-
Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS.Neuron. 2011 Oct 20;72(2):245-56. doi: 10.1016/j.neuron.2011.09.011. Epub 2011 Sep 21. Neuron. 2011. PMID: 21944778 Free PMC article.
-
C9orf72-Associated Arginine-Rich Dipeptide Repeat Proteins Reduce the Number of Golgi Outposts and Dendritic Branches in Drosophila Neurons.Mol Cells. 2020 Sep 30;43(9):821-830. doi: 10.14348/molcells.2020.0130. Mol Cells. 2020. PMID: 32975212 Free PMC article.
References
-
- Harding A.E. The clinical features and classification of the late onset autosomal dominant cerebellar ataxias. A study of 11 families, including descendants of the ‘the Drew family of Walworth’. Brain. 1982;105:1–28. - PubMed
-
- Matilla-Dueñas A., Sánchez I., Corral-Juan M., Dávalos A., Alvarez R., Latorre P. Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum. 2010;9:148–166. - PubMed
-
- Schöls L., Bauer P., Schmidt T., Schulte T., Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304. - PubMed
-
- Ohta Y., Hayashi T., Nagai M., Okamoto M., Nagotani S., Nagano I., Ohmori N., Takehisa Y., Murakami T., Shoji M. Two cases of spinocerebellar ataxia accompanied by involvement of the skeletal motor neuron system and bulbar palsy. Intern. Med. 2007;46:751–755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

