Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study
- PMID: 21684583
- PMCID: PMC3152667
- DOI: 10.1016/j.ygyno.2011.05.040
Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study
Abstract
Purpose: The Gynecologic Oncology Group (GOG) conducted a phase II trial to assess the efficacy and tolerability of the anti-EGFR antibody cetuximab, in persistent or recurrent carcinoma of the cervix.
Patients and methods: Eligible patients had cervical cancer, measurable disease, and GOG performance status ≤2. Treatment consisted of cetuximab 400 mg/m(2) initial dose followed by 250 mg/m(2) weekly until disease progression or prohibitive toxicity. The primary endpoints were progression-free survival (PFS) at 6 months and response. The study used a 2-stage group sequential design.
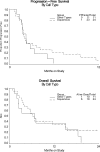
Results: Thirty-eight patients were entered with 3 exclusions, leaving 35 evaluable for analysis. Thirty-one patients (88.6%) received prior radiation as well as either 1 (n=25, 71.4%) or 2 (n=10) prior cytotoxic regimens. Twenty-four patients (68.6%) had a squamous cell carcinoma. Grade 3 adverse events possibly related to cetuximab included dermatologic (n=5), GI (n=4), anemia (n=2), constitutional (n=3), infection (n=2), vascular (n=2), pain (n=2), and pulmonary, neurological, vomiting and metabolic (n=1 each). No clinical responses were detected. Five patients (14.3%; two-sided 90% CI, 5.8% to 30%) survived without progression for at least 6 months. The median PFS and overall survival (OS) times were 1.97 and 6.7 months, respectively. In this study, all patients with PFS at 6 months harbored tumors with squamous cell histology.
Conclusion: Cetuximab is well tolerated but has limited activity in this population. Cetuximab activity may be limited to patients with squamous cell histology.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study.J Clin Oncol. 2009 Mar 1;27(7):1069-74. doi: 10.1200/JCO.2008.18.9043. Epub 2009 Jan 12. J Clin Oncol. 2009. PMID: 19139430 Free PMC article. Clinical Trial.
-
A phase II evaluation of brivanib in the treatment of persistent or recurrent carcinoma of the cervix: An NRG Oncology/Gynecologic Oncology Group study.Gynecol Oncol. 2017 Sep;146(3):554-559. doi: 10.1016/j.ygyno.2017.05.033. Epub 2017 Jul 18. Gynecol Oncol. 2017. PMID: 28728751 Free PMC article. Clinical Trial.
-
Phase II study of cisplatin plus cetuximab in advanced, recurrent, and previously treated cancers of the cervix and evaluation of epidermal growth factor receptor immunohistochemical expression: a Gynecologic Oncology Group study.Gynecol Oncol. 2011 May 1;121(2):303-8. doi: 10.1016/j.ygyno.2011.01.030. Epub 2011 Feb 16. Gynecol Oncol. 2011. PMID: 21329967 Free PMC article. Clinical Trial.
-
A randomized phase II study of cetuximab every 2 weeks at either 500 or 750 mg/m2 for patients with recurrent or metastatic head and neck squamous cell cancer.J Natl Compr Canc Netw. 2012 Nov 1;10(11):1391-8. doi: 10.6004/jnccn.2012.0144. J Natl Compr Canc Netw. 2012. PMID: 23138167 Free PMC article. Clinical Trial.
-
Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study.J Clin Oncol. 2007 Nov 20;25(33):5165-71. doi: 10.1200/JCO.2007.11.5345. J Clin Oncol. 2007. PMID: 18024863 Clinical Trial.
Cited by
-
Integrated analysis of EREG expression, a gene associated with cervical cancer prognosis.Am J Cancer Res. 2023 Oct 15;13(10):4644-4660. eCollection 2023. Am J Cancer Res. 2023. PMID: 37970371 Free PMC article.
-
Targeted therapies in gynecological cancers: a comprehensive review of clinical evidence.Signal Transduct Target Ther. 2020 Jul 29;5(1):137. doi: 10.1038/s41392-020-0199-6. Signal Transduct Target Ther. 2020. PMID: 32728057 Free PMC article. Review.
-
Updates in systemic treatment for metastatic cervical cancer.Curr Treat Options Oncol. 2014 Mar;15(1):1-13. doi: 10.1007/s11864-013-0273-1. Curr Treat Options Oncol. 2014. PMID: 24429797 Review.
-
An EGFR-Amplified Cervical Squamous Cell Carcinoma Patient with Pulmonary Metastasis Benefits from Afatinib: A Case Report.Onco Targets Ther. 2020 Feb 28;13:1845-1849. doi: 10.2147/OTT.S236382. eCollection 2020. Onco Targets Ther. 2020. PMID: 32184619 Free PMC article.
-
A 70-Gene Signature for Predicting Treatment Outcome in Advanced-Stage Cervical Cancer.Mol Ther Oncolytics. 2020 Sep 5;19:47-56. doi: 10.1016/j.omto.2020.09.001. eCollection 2020 Dec 16. Mol Ther Oncolytics. 2020. PMID: 33024818 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomized study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–40. - PubMed
-
- Arteaga CL. Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol. 2002;29:3–9. - PubMed
-
- Baselga J. New therapeutic agents targeting the epidermal growth factor receptor. J Clin Oncol. 2000;18:54S–59S. - PubMed
-
- Raymond E, Faivre S, Armand JP. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs. 2000;60:15–23. discussion, 41–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous