Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study
- PMID: 21684583
- PMCID: PMC3152667
- DOI: 10.1016/j.ygyno.2011.05.040
Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study
Abstract
Purpose: The Gynecologic Oncology Group (GOG) conducted a phase II trial to assess the efficacy and tolerability of the anti-EGFR antibody cetuximab, in persistent or recurrent carcinoma of the cervix.
Patients and methods: Eligible patients had cervical cancer, measurable disease, and GOG performance status ≤2. Treatment consisted of cetuximab 400 mg/m(2) initial dose followed by 250 mg/m(2) weekly until disease progression or prohibitive toxicity. The primary endpoints were progression-free survival (PFS) at 6 months and response. The study used a 2-stage group sequential design.
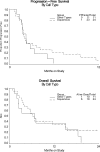
Results: Thirty-eight patients were entered with 3 exclusions, leaving 35 evaluable for analysis. Thirty-one patients (88.6%) received prior radiation as well as either 1 (n=25, 71.4%) or 2 (n=10) prior cytotoxic regimens. Twenty-four patients (68.6%) had a squamous cell carcinoma. Grade 3 adverse events possibly related to cetuximab included dermatologic (n=5), GI (n=4), anemia (n=2), constitutional (n=3), infection (n=2), vascular (n=2), pain (n=2), and pulmonary, neurological, vomiting and metabolic (n=1 each). No clinical responses were detected. Five patients (14.3%; two-sided 90% CI, 5.8% to 30%) survived without progression for at least 6 months. The median PFS and overall survival (OS) times were 1.97 and 6.7 months, respectively. In this study, all patients with PFS at 6 months harbored tumors with squamous cell histology.
Conclusion: Cetuximab is well tolerated but has limited activity in this population. Cetuximab activity may be limited to patients with squamous cell histology.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomized study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–40. - PubMed
-
- Arteaga CL. Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol. 2002;29:3–9. - PubMed
-
- Baselga J. New therapeutic agents targeting the epidermal growth factor receptor. J Clin Oncol. 2000;18:54S–59S. - PubMed
-
- Raymond E, Faivre S, Armand JP. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs. 2000;60:15–23. discussion, 41–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous