Evidence for host cells as the major contributor of lipids in the intravacuolar network of Toxoplasma-infected cells
- PMID: 21685319
- PMCID: PMC3165450
- DOI: 10.1128/EC.00002-11
Evidence for host cells as the major contributor of lipids in the intravacuolar network of Toxoplasma-infected cells
Abstract
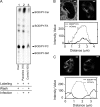
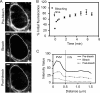
The intracellular parasite Toxoplasma gondii develops inside a parasitophorous vacuole (PV) that derives from the host cell plasma membrane during invasion. Previous electron micrograph images have shown that the membrane of this vacuole undergoes an extraordinary remodeling with an extensive network of thin tubules and vesicles, the intravacuolar network (IVN), which fills the lumen of the PV. While dense granule proteins, secreted during and after invasion, are the main factors for the organization and tubulation of the network, little is known about the source of lipids used for this remodeling. By selectively labeling host cell or parasite membranes, we uncovered evidence that strongly supports the host cell as the primary, if not exclusive, source of lipids for parasite IVN remodeling. Fluorescence recovery after photobleaching (FRAP) microscopy experiments revealed that lipids are surprisingly dynamic within the parasitophorous vacuole and are continuously exchanged or replenished by the host cell. The results presented here suggest a new model for development of the parasitophorous vacuole whereby the host provides a continuous stream of lipids to support the growth and maturation of the PVM and IVN.
Figures
References
-
- Bai J., Pagano R. E. 1997. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry 36:8840–8848 - PubMed
-
- Charron A. J., Sibley L. D. 2002. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 115:3049–3059 - PubMed
-
- Coppens I., et al. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125:261–274 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

