Biological mechanisms, one molecule at a time
- PMID: 21685361
- PMCID: PMC3127425
- DOI: 10.1101/gad.2050011
Biological mechanisms, one molecule at a time
Abstract
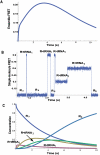
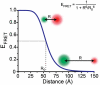
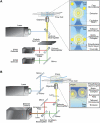
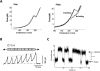
The last 15 years have witnessed the development of tools that allow the observation and manipulation of single molecules. The rapidly expanding application of these technologies for investigating biological systems of ever-increasing complexity is revolutionizing our ability to probe the mechanisms of biological reactions. Here, we compare the mechanistic information available from single-molecule experiments with the information typically obtained from ensemble studies and show how these two experimental approaches interface with each other. We next present a basic overview of the toolkit for observing and manipulating biology one molecule at a time. We close by presenting a case study demonstrating the impact that single-molecule approaches have had on our understanding of one of life's most fundamental biochemical reactions: the translation of a messenger RNA into its encoded protein by the ribosome.
Figures



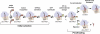

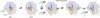



References
-
- Aitken CE, Petrov A, Puglisi JD 2010. Single ribosome dynamics and the mechanism of translation. Annu Rev Biophys 39: 491–513 - PubMed
-
- Ashkin A 1970. Acceleration and trapping of particles by radiation pressure. Phys Rev Lett 24: 156–159
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
