Plasmodium falciparum uses a key functional site in complement receptor type-1 for invasion of human erythrocytes
- PMID: 21685372
- PMCID: PMC3158720
- DOI: 10.1182/blood-2011-03-341305
Plasmodium falciparum uses a key functional site in complement receptor type-1 for invasion of human erythrocytes
Abstract
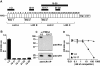
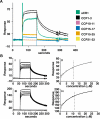
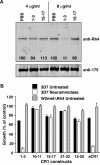
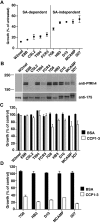
The Plasmodium falciparum adhesin PfRh4 binds to complement receptor type-1 (CR1) on human erythrocytes and mediates a glycophorin-independent invasion pathway. CR1 is a complement regulator and immune-adherence receptor on erythrocytes required for shuttling of C3b/C4b-opsonized particles to liver and spleen for phagocytosis. Using recombinant CR1 constructs, we mapped the recognition site for PfRh4 to complement control protein modules 1 to 3 (CCP1-3) at the membrane-distal amino terminus of CR1. This region of CR1 binds to C4b and C3b and accelerates decay of both classic pathway and alternative pathway C3 and C5 convertases. CCP1-3 competed for PfRh4 binding to erythroid CR1 and inhibited the PfRh4-CR1 invasion pathways across a wide range of P falciparum strains. PfRh4 did not bind significantly to other CR1 constructs, including CCP15-17, which is 85% identical to CCP1-3. PfRh4 binding to CR1 did not affect its C3b/C4b binding capability, and we show evidence for a ternary complex between CCP1-3, C4b, and PfRh4. PfRh4 binding specifically inhibited CR1's convertase decay-accelerating activity, whereas there was no effect on factor H-mediated decay-accelerating activity. These results increase our understanding of the functional implications of CR1 engagement with PfRh4 and highlight the interplay between complement regulation and infection.
Figures



References
-
- Hourcade D, Holers VM, Atkinson JP. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. - PubMed
-
- Dörig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993;75(2):295–305. - PubMed
-
- Speth C, Kacani L, Dierich MP. Complement receptors in HIV infection. Immunol Rev. 1997;159:49–67. - PubMed
-
- Schorey JS, Carroll MC, Brown EJ. A macrophage invasion mechanism of pathogenic mycobacteria. Science. 1997;277(5329):1091–1093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

