G-CSF supplementation with chemotherapy can promote revascularization and subsequent tumor regrowth: prevention by a CXCR4 antagonist
- PMID: 21685373
- PMCID: PMC3179406
- DOI: 10.1182/blood-2010-11-320812
G-CSF supplementation with chemotherapy can promote revascularization and subsequent tumor regrowth: prevention by a CXCR4 antagonist
Abstract
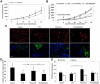
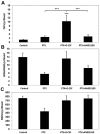
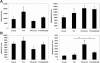
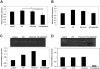
Recombinant granulocyte colony-stimulating factor (G-CSF) is used to accelerate recovery from chemotherapy-induced myelosuppression. G-CSF has been recently shown to stimulate angiogenesis mediated by several types of bone marrow-derived cell populations. To investigate whether G-CSF may alter tumor response to therapy, we studied Lewis lung and EMT/6 breast carcinomas in mice treated with paclitaxel (PTX) chemotherapy in combination with G-CSF. We compared the results obtained to mice treated with PTX and AMD3100, a small-molecule drug antagonist of CXCR4 which, like G-CSF, can be used to mobilize hematopoietic cells. We show that PTX combined with G-CSF treatment facilitates revascularization, leading to an improvement in blood perfusion in LLC tumors, and a decrease in hypoxia in EMT/6 tumors, thus enhancing tumor growth in comparison to PTX or PTX and AMD3100 therapies. We found that hemangiocytes but not Gr-1(+) CD11b(+) cells colonize EMT/6 tumors after treatment with PTX and G-CSF, but not PTX and AMD3100, and therefore may contribute to angiogenesis. However, increases in hemangiocyte colonization were not observed in LLC PTX and G-CSF-treated tumors, suggesting distinct mechanisms of tumor revascularization after G-CSF. Overall, our observations suggest that despite its known considerable clinical benefits, G-CSF might contribute to tumor revascularization by various mechanisms, and diminish the antitumor activity of chemotherapy, an effect that can be prevented by AMD3100.
Figures


References
-
- Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. - PubMed
-
- Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5(7):516–525. - PubMed
-
- Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20(24):4713–4721. - PubMed
-
- Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–3205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

