Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial
- PMID: 21685437
- PMCID: PMC3117310
- DOI: 10.1136/bmj.d3527
Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial
Abstract
Objective: To evaluate efficacy and safety outcomes in patients in the PLATelet inhibition and patient Outcomes (PLATO) trial who at randomisation were planned for a non-invasive treatment strategy.
Design: Pre-specified analysis of pre-randomisation defined subgroup of prospective randomised clinical trial.
Setting: 862 centres in 43 countries.
Participants: 5216 (28%) of 18,624 patients admitted to hospital for acute coronary syndrome who were specified as planned for non-invasive management.
Interventions: Randomised treatment with ticagrelor (n=2601) versus clopidogrel (2615).
Main outcome measurements: Primary composite end point of cardiovascular death, myocardial infarction, and stroke; their individual components; and PLATO defined major bleeding during one year.
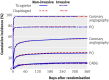
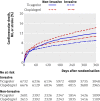
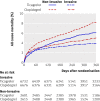
Results: 2183 (41.9%) patients had coronary angiography during their initial hospital admission, 1065 (20.4%) had percutaneous coronary intervention, and 208 (4.0%) had coronary artery bypass surgery. Cumulatively, 3143 (60.3%) patients had been managed non-invasively by the end of follow-up. The incidence of the primary end point was lower with ticagrelor than with clopidogrel (12.0% (n=295) v 14.3% (346); hazard ratio 0.85, 95% confidence interval 0.73 to 1.00; P=0.04). Overall mortality was also lower (6.1% (147) v 8.2% (195); 0.75, 0.61 to 0.93; P=0.01). The incidence of total major bleeding (11.9% (272) v 10.3% (238); 1.17, 0.98 to 1.39; P=0.08) and non-coronary artery bypass grafting related major bleeding (4.0% (90) v 3.1% (71); 1.30, 0.95 to 1.77; P=0.10) was numerically higher with ticagrelor than with clopidogrel.
Conclusions: In patients with acute coronary syndrome initially intended for non-invasive management, the benefits of ticagrelor over clopidogrel were consistent with those from the overall PLATO results, indicating the broad benefits of P2Y12 inhibition with ticagrelor regardless of intended management strategy.
Trial registration: Clinical trials NCT00391872.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures

Comment in
-
Non-interventional management of acute coronary syndromes.BMJ. 2011 Jun 17;342:d3263. doi: 10.1136/bmj.d3263. BMJ. 2011. PMID: 21685435 No abstract available.
References
-
- Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007;28:1598-660. - PubMed
-
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation 2007;116:e148-304. - PubMed
-
- De Winter RJ, Windhausen F, Cornel JH, Dunselman PH, Janus CL, Bendermacher PE, et al. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med 2005;353:1095-104. - PubMed
-
- Chan MY, Becker RC, Harrington RA, Peterson ED, Armstrong PW, White H, et al. Noninvasive, medical management for non-ST-elevation acute coronary syndromes. Am Heart J 2008;155:397-407. - PubMed
-
- Amsterdam EA, Peterson ED, Ou FS, Newby LK, Pollack CV Jr, Gibler WB, et al. Comparative trends in guidelines adherence among patients with non-ST-segment elevation acute coronary syndromes treated with invasive versus conservative management strategies: results from the CRUSADE quality improvement initiative. Am Heart J 2009;158:748-54. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
