S1 ribosomal protein and the interplay between translation and mRNA decay
- PMID: 21685451
- PMCID: PMC3177188
- DOI: 10.1093/nar/gkr417
S1 ribosomal protein and the interplay between translation and mRNA decay
Abstract
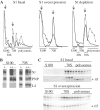
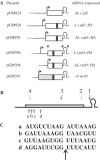
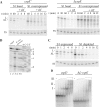
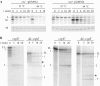
S1 is an 'atypical' ribosomal protein weakly associated with the 30S subunit that has been implicated in translation, transcription and control of RNA stability. S1 is thought to participate in translation initiation complex formation by assisting 30S positioning in the translation initiation region, but little is known about its role in other RNA transactions. In this work, we have analysed in vivo the effects of different intracellular S1 concentrations, from depletion to overexpression, on translation, decay and intracellular distribution of leadered and leaderless messenger RNAs (mRNAs). We show that the cspE mRNA, like the rpsO transcript, may be cleaved by RNase E at multiple sites, whereas the leaderless cspE transcript may also be degraded via an alternative pathway by an unknown endonuclease. Upon S1 overexpression, RNase E-dependent decay of both cspE and rpsO mRNAs is suppressed and these transcripts are stabilized, whereas cleavage of leaderless cspE mRNA by the unidentified endonuclease is not affected. Overall, our data suggest that ribosome-unbound S1 may inhibit translation and that part of the Escherichia coli ribosomes may actually lack S1.
Figures

References
-
- Kaberdin VR, Bläsi U. Translation initiation and the fate of bacterial mRNAs. FEMS Microbiol. Rev. 2006;30:967–979. - PubMed
-
- Deana A, Belasco JG. Lost in translation: the influence of ribosomes on bacterial mRNA decay. Genes Dev. 2005;19:2526–2533. - PubMed
-
- Kalapos MP, Paulus H, Sarkar N. Identification of ribosomal protein S1 as a poly(A) binding protein in Escherichia coli. Biochimie. 1997;79:493–502. - PubMed
-
- Feng Y, Huang H, Liao J, Cohen SN. Escherichia coli poly(A)-binding proteins that interact with components of degradosomes or impede RNA decay mediated by polynucleotide phosphorylase and RNase E. J. Biol. Chem. 2001;276:31651–31656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

