Multiple oligomerization domains of KANK1-PDGFRβ are required for JAK2-independent hematopoietic cell proliferation and signaling via STAT5 and ERK
- PMID: 21685469
- PMCID: PMC3186300
- DOI: 10.3324/haematol.2011.040147
Multiple oligomerization domains of KANK1-PDGFRβ are required for JAK2-independent hematopoietic cell proliferation and signaling via STAT5 and ERK
Abstract
Background: KANK1-PDGFRB is a fusion gene generated by the t(5;9) translocation between KANK1 and the platelet-derived growth factor receptor beta gene PDGFRB. This hybrid was identified in a myeloproliferative neoplasm featuring severe thrombocythemia, in the absence of the JAK2 V617F mutation.
Design and methods: KANK1-PDGFRB was transduced into Ba/F3 cells and CD34(+) human progenitor cells to gain insights into the mechanisms whereby this fusion gene transforms cells.
Results: Although platelet-derived growth factor receptors are capable of activating JAK2, KANK1-PDGFRβ did not induce JAK2 phosphorylation in hematopoietic cells and a JAK inhibitor did not affect KANK1-PDGFRβ-induced cell growth. Like JAK2 V617F, KANK1-PDGFRβ constitutively activated STAT5 transcription factors, but this did not require JAK kinases. In addition KANK1-PDGFRβ induced the phosphorylation of phospholipase C-γ, ERK1 and ERK2, like wild-type PDGFRβ and TEL-PDGFRβ, another hybrid protein found in myeloid malignancies. We next tested various mutant forms of KANK1-PDGFRβ in Ba/F3 cells and human CD34(+) hematopoietic progenitors. The three coiled-coil domains located in the N-terminus of KANK1 were required for KANK1-PDGFRβ-induced cell growth and signaling via STAT5 and ERK. However, the coiled-coils were not essential for KANK1-PDGFRβ oligomerization, which could be mediated by another new oligomerization domain. KANK1-PDGFRβ formed homotrimeric complexes and heavier oligomers.
Conclusions: KANK1-PDGFRB is a unique example of a thrombocythemia-associated oncogene that does not signal via JAK2. The fusion protein is activated by multiple oligomerization domains, which are required for signaling and cell growth stimulation.
Figures

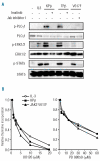




Similar articles
-
Tel/PDGFRbeta induces stem cell differentiation via the Ras/ERK and STAT5 signaling pathways.Exp Hematol. 2009 Jan;37(1):111-121. doi: 10.1016/j.exphem.2008.09.012. Exp Hematol. 2009. PMID: 19100521
-
Ectopic PD-L1 expression in JAK2 (V617F) myeloproliferative neoplasm patients is mediated via increased activation of STAT3 and STAT5.Hum Cell. 2020 Oct;33(4):1099-1111. doi: 10.1007/s13577-020-00370-6. Epub 2020 Jul 14. Hum Cell. 2020. PMID: 32430672
-
STAT5 activation is critical for the transformation mediated by myeloproliferative disorder-associated JAK2 V617F mutant.J Biol Chem. 2010 Feb 19;285(8):5296-307. doi: 10.1074/jbc.M109.040733. Epub 2009 Dec 22. J Biol Chem. 2010. PMID: 20028972 Free PMC article.
-
[Analysis of oncogenic signaling pathway induced by a myeloproliferative neoplasm-associated Janus kinase 2 (JAK2) V617F mutant].Yakugaku Zasshi. 2012;132(11):1267-72. doi: 10.1248/yakushi.12-00225. Yakugaku Zasshi. 2012. PMID: 23123718 Review. Japanese.
-
Myeloid neoplasms with eosinophilia.Blood. 2017 Feb 9;129(6):704-714. doi: 10.1182/blood-2016-10-695973. Epub 2016 Dec 27. Blood. 2017. PMID: 28028030 Review.
Cited by
-
Tyrosine kinase gene fusions in cancer: translating mechanisms into targeted therapies.J Cell Mol Med. 2012 Feb;16(2):237-48. doi: 10.1111/j.1582-4934.2011.01415.x. J Cell Mol Med. 2012. PMID: 21854543 Free PMC article. Review.
-
PDGFRB mutants found in patients with familial infantile myofibromatosis or overgrowth syndrome are oncogenic and sensitive to imatinib.Oncogene. 2016 Jun 23;35(25):3239-48. doi: 10.1038/onc.2015.383. Epub 2015 Oct 12. Oncogene. 2016. PMID: 26455322
-
The tyrosine phosphatase SHP2 is required for cell transformation by the receptor tyrosine kinase mutants FIP1L1-PDGFRα and PDGFRα D842V.Mol Oncol. 2014 May;8(3):728-40. doi: 10.1016/j.molonc.2014.02.003. Epub 2014 Feb 17. Mol Oncol. 2014. PMID: 24618081 Free PMC article.
-
PDGF: the nuts and bolts of signalling toolbox.Tumour Biol. 2011 Dec;32(6):1057-70. doi: 10.1007/s13277-011-0212-3. Epub 2011 Jul 19. Tumour Biol. 2011. PMID: 21769672 Review.
-
PDGF-induced fibroblast growth requires monounsaturated fatty acid production by stearoyl-CoA desaturase.FEBS Open Bio. 2017 Feb 2;7(3):414-423. doi: 10.1002/2211-5463.12194. eCollection 2017 Mar. FEBS Open Bio. 2017. PMID: 28286737 Free PMC article.
References
-
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. - PubMed
-
- Staerk J, Kallin A, Royer Y, Diaconu CC, Dusa A, Demoulin JB, et al. JAK2, the JAK2 V617F mutant and cytokine receptors. Pathol Biol (Paris) 2007;55(2):88–91. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. - PubMed
-
- Toffalini F, Demoulin JB. New insights into the mechanisms of hematopoietic cell transformation by activated receptor tyrosine kinases. Blood. 2010;116(14):2429–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

