Coordination of cell cycle, DNA repair and muscle gene expression in myoblasts exposed to genotoxic stress
- PMID: 21685725
- PMCID: PMC3322469
- DOI: 10.4161/cc.10.14.15948
Coordination of cell cycle, DNA repair and muscle gene expression in myoblasts exposed to genotoxic stress
Abstract
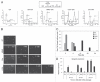
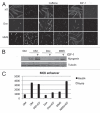
Upon exposure to genotoxic stress, skeletal muscle progenitors coordinate DNA repair and the activation of the differentiation program through the DNA damage-activated differentiation checkpoint, which holds the transcription of differentiation genes while the DNA is repaired. A conceptual hurdle intrinsic to this process relates to the coordination of DNA repair and muscle-specific gene transcription within specific cell cycle boundaries (cell cycle checkpoints) activated by different types of genotoxins. Here, we show that, in proliferating myoblasts, the inhibition of muscle gene transcription occurs by either a G 1- or G 2-specific differentiation checkpoint. In response to genotoxins that induce G 1 arrest, MyoD binds target genes but is functionally inactivated by a c-Abl-dependent phosphorylation. In contrast, DNA damage-activated G 2 checkpoint relies on the inability of MyoD to bind the chromatin at the G 2 phase of the cell cycle. These results indicate an intimate relationship between DNA damage-activated cell cycle checkpoints and the control of tissue-specific gene expression to allow DNA repair in myoblasts prior to the activation of the differentiation program.
Figures


Comment in
-
Checking before changing: cell cycle checkpoints inhibit muscle differentiation.Cell Cycle. 2011 Oct 1;10(19):3234-5. doi: 10.4161/cc.10.19.17127. Epub 2011 Oct 1. Cell Cycle. 2011. PMID: 21946520 Free PMC article. No abstract available.
Similar articles
-
DNA damage-activated ABL-MyoD signaling contributes to DNA repair in skeletal myoblasts.Cell Death Differ. 2013 Dec;20(12):1664-74. doi: 10.1038/cdd.2013.118. Epub 2013 Sep 20. Cell Death Differ. 2013. PMID: 24056763 Free PMC article.
-
A myogenic differentiation checkpoint activated by genotoxic stress.Nat Genet. 2002 Dec;32(4):585-93. doi: 10.1038/ng1023. Epub 2002 Nov 4. Nat Genet. 2002. PMID: 12415271
-
An evolutionarily acquired genotoxic response discriminates MyoD from Myf5, and differentially regulates hypaxial and epaxial myogenesis.EMBO Rep. 2011 Feb;12(2):164-71. doi: 10.1038/embor.2010.195. Epub 2011 Jan 7. EMBO Rep. 2011. PMID: 21212806 Free PMC article.
-
Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts.Cell Mol Life Sci. 2001 Apr;58(4):571-9. doi: 10.1007/PL00000882. Cell Mol Life Sci. 2001. PMID: 11361092 Free PMC article. Review.
-
Determination of cell fate by c-Abl activation in the response to DNA damage.Oncogene. 1998 Dec 24;17(25):3309-18. doi: 10.1038/sj.onc.1202571. Oncogene. 1998. PMID: 9916993 Review.
Cited by
-
RNF138 regulates skeletal muscle differentiation via the Wnt/β-catenin signaling pathway.Theranostics. 2025 Mar 18;15(10):4446-4464. doi: 10.7150/thno.110925. eCollection 2025. Theranostics. 2025. PMID: 40225576 Free PMC article.
-
Muscle gets stressed? p53 represses and protects.Cell Death Differ. 2015 Apr;22(4):519-21. doi: 10.1038/cdd.2014.223. Cell Death Differ. 2015. PMID: 25747852 Free PMC article. No abstract available.
-
The Spindle Assembly Checkpoint Safeguards Genomic Integrity of Skeletal Muscle Satellite Cells.Stem Cell Reports. 2015 Jun 9;4(6):1061-74. doi: 10.1016/j.stemcr.2015.04.006. Epub 2015 May 7. Stem Cell Reports. 2015. PMID: 25960061 Free PMC article.
-
Checking before changing: cell cycle checkpoints inhibit muscle differentiation.Cell Cycle. 2011 Oct 1;10(19):3234-5. doi: 10.4161/cc.10.19.17127. Epub 2011 Oct 1. Cell Cycle. 2011. PMID: 21946520 Free PMC article. No abstract available.
-
DNA damage signaling mediates the functional antagonism between replicative senescence and terminal muscle differentiation.Genes Dev. 2017 Apr 1;31(7):648-659. doi: 10.1101/gad.293266.116. Genes Dev. 2017. PMID: 28446595 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
